Gene-based therapy for the treatment of spinal muscular atrophy types 1 and 2 : a systematic review and meta-analysis
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
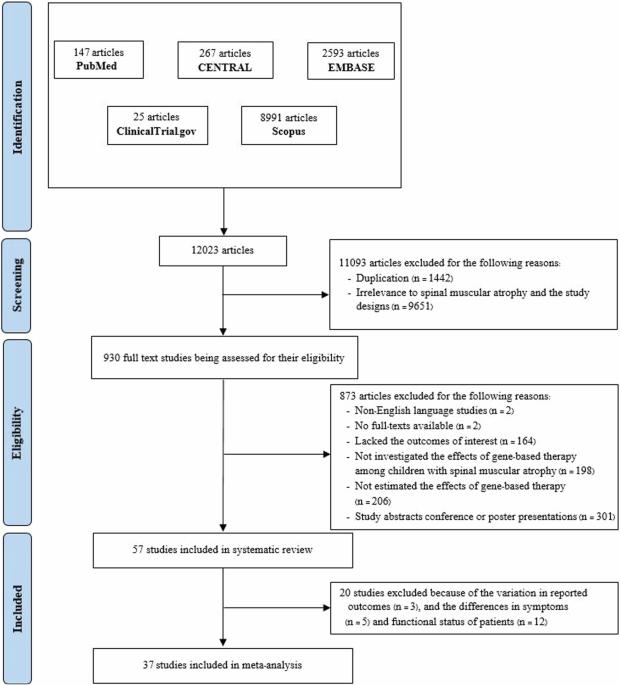
Despite numerous studies identifying the advantages of therapies for spinal muscular atrophy (SMA), healthcare professionals encounter obstacles in determining the most effective treatment. This study aimed to investigate the effects of gene-based therapy for SMA. A systematic search was conducted from inception to May 2024 across databases, and all studies assessing the effects of gene-based therapy on patients with SMA types 1 and 2 were included. The outcomes measured were survival, the need for ventilatory support, improvements in motor function, and the occurrence of adverse drug reactions. Meta-analyses were performed using a random-effects model. A total of 57 studies (n = 3418) were included, and the meta-analyses revealed that onasemnogene abeparvovec showed the highest survival rate (95% [95% CI: 88, 100]), followed by risdiplam (86% [95% CI: 76, 94]) and nusinersen (60% [95% CI: 50, 70]). The number of patients needing ventilatory support was reduced after treatment with onasemnogene abeparvovec (risk ratio = 0·10 [95% CI: 0·02, 0·53]). Onasemnogene abeparvovec and risdiplam had similar proportions of patients with improvements in the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders score of ≥4 points (92% [95% CI: 62, 100] vs 90% [95% CI: 77, 97]). In contrast, nusinersen had the smallest improvement (74% [95% CI: 66, 81]). The most frequently observed adverse drug reactions were headaches, vomiting, and gastrointestinal disorders. Gene-based therapy benefits patient survival and improves motor function. Onasemnogene abeparvovec and risdiplam appear highly effective, whereas nusinersen exhibits moderate effectiveness.
治疗脊髓性肌萎缩症 1 型和 2 型的基因疗法:系统综述和荟萃分析。
尽管有大量研究证实了脊髓性肌萎缩症(SMA)疗法的优势,但医护人员在确定最有效的治疗方法时仍会遇到障碍。本研究旨在调查基于基因的疗法对 SMA 的治疗效果。从开始到2024年5月,我们在各数据库中进行了系统性检索,纳入了所有评估基因疗法对1型和2型SMA患者影响的研究。研究结果包括存活率、呼吸支持需求、运动功能改善情况以及药物不良反应发生率。元分析采用随机效应模型进行。荟萃分析显示,onasemnogene abeparvovec的存活率最高(95% [95% CI:88, 100]),其次是risdiplam(86% [95% CI:76, 94])和nusinersen(60% [95% CI:50, 70])。使用onasemnogene abeparvovec治疗后,需要呼吸机支持的患者人数有所减少(风险比=0-10 [95% CI:0-02,0-53])。费城儿童医院婴儿神经肌肉障碍测试评分≥4分的患者比例与onasemnogene abeparvovec和risdiplam相似(92% [95% CI: 62, 100] vs 90% [95% CI: 77, 97])。相比之下,纽西奈森的改善幅度最小(74% [95% CI:66, 81])。最常见的药物不良反应是头痛、呕吐和胃肠功能紊乱。基于基因的疗法有利于患者存活并改善运动功能。Onasemnogene abeparvovec 和 risdiplam 显得非常有效,而 nusinersen 则表现出中等疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


