PGAM5 promotes RIPK1-PANoptosome activity by phosphorylating and activating RIPK1 to mediate PANoptosis after subarachnoid hemorrhage in rats
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Neuronal death plays a crucial role in early brain injury after subarachnoid hemorrhage (SAH). PANoptosis is a programmed form of cell death regulated by the PANoptosome, which possesses key characteristics of pyroptosis, apoptosis and necroptosis. Phosphoglycerate mutase family member 5 (PGAM5) has specific phosphatase activity that phosphorylates or dephosphorylates serine and threonine residues on bound proteins such as receptor-interacting protein kinase 1 (RIPK1), which are involved in programmed cell death. This study aimed to explore whether PANoptosis occurs after subarachnoid hemorrhage and to investigate the role of PGAM5 in early brain injury after SAH. A monofilament perforation SAH model in Sprague–Dawley rats was established, and PGAM5 siRNA (siPGAM5) was administered via intracerebroventricular injection 48 h before SAH modeling. The efficacy of siPGAM5 treatment was assessed via neurological scoring, and the impact of siPGAM5 on PANoptosis was evaluated via Western blotting, TUNEL staining and ELISA. To investigate its potential mechanism, the RIPK1 activator birinapant was administered intraperitoneally 0.5 h after SAH. The role of RIPK in PGAM5-mediated PANoptosis was evaluated by Western blotting and coimmunoprecipitation. Our findings indicate that PANoptosis occurs in neurons after SAH and that reducing PGAM5 in the cytosol after SAH can reduce PANoptosis and enhance the short-term and long-term neurological functions of SAH rats. Mechanistically, we discovered that PGAM5 can directly bind to and phosphorylate and activate RIPK1 (ser 166), triggering the assembly of the RIPK1-PANoptosome complex. In conclusion, our study revealed that the increased PGAM5 in the mitochondria-free cytosol after SAH can bind to and activate RIPK1 (ser 166), driving the assembly of the RIPK1-PANoptosome and mediating PANoptosis after SAH. PGAM5 and PANoptosis might be novel therapeutic targets for SAH.
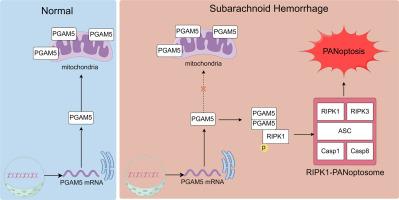
PGAM5通过磷酸化和激活RIPK1促进RIPK1-PANoptosome活性,从而介导大鼠蛛网膜下腔出血后的PAN凋亡。
神经元死亡在蛛网膜下腔出血(SAH)后的早期脑损伤中起着至关重要的作用。PANoptosis是由PANoptosome调控的一种程序性细胞死亡形式,它具有热凋亡、细胞凋亡和坏死的主要特征。磷酸甘油酸突变酶家族成员5(PGAM5)具有特异性磷酸酶活性,能使受体相互作用蛋白激酶1(RIPK1)等结合蛋白上的丝氨酸和苏氨酸残基磷酸化或去磷酸化,这些蛋白参与了细胞的程序性死亡。本研究旨在探讨蛛网膜下腔出血后是否会发生 PAN 细胞凋亡,并研究 PGAM5 在 SAH 后早期脑损伤中的作用。研究建立了单丝穿孔SAH模型,并在SAH模型建立前48小时通过脑室内注射PGAM5 siRNA(siPGAM5)。siPGAM5 的疗效通过神经系统评分进行评估,而 siPGAM5 对 PAN 凋亡的影响则通过 Western 印迹、TUNEL 染色和 ELISA 进行评估。为了研究其潜在机制,在 SAH 后 0.5 小时腹腔注射 RIPK1 激活剂 birinapant。通过 Western 印迹和共沉淀法评估了 RIPK 在 PGAM5 介导的 PAN 细胞凋亡中的作用。我们的研究结果表明,SAH 后神经元会发生 PANoptosis,而减少 SAH 后细胞质中的 PGAM5 可以减少 PANoptosis 并增强 SAH 大鼠的短期和长期神经功能。从机理上讲,我们发现 PGAM5 可直接与 RIPK1 结合并磷酸化和激活 RIPK1(ser 166),触发 RIPK1-PANoptosome 复合物的组装。总之,我们的研究揭示了 SAH 后线粒体无细胞质中增加的 PGAM5 可与 RIPK1(ser 166)结合并激活 RIPK1(ser 166),从而驱动 RIPK1-PANoptosome 的组装并介导 SAH 后的 PANoptosis。PGAM5和PAN凋亡可能是治疗SAH的新靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


