Solubility behavior of Rosiglitazone in ten pure solvents: Model correlation, thermodynamic calculations, and molecular simulation
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Rosiglitazone is an oral antidiabetic medication primarily used to treat type 2 diabetes. Herein, the single crystal structure of Rosiglitazone (a = 14.0613(7) Å, b = 8.4675(4) Å, c = 29.8841(16) Å, space group Pbca, Z = 8, V = 3558.1(3) Å3) was analyzed for the first time. Through solubility measurements, model correlation, thermodynamic calculations, and molecular simulations, the dissolution of Rosiglitazone in ten pure solvents was comprehensively investigated. The solubility order at 298.15 K is acetone > methyl acetate > ethyl acetate > butyl acetate > acetonitrile > alcoholic solvents (methanol, ethanol, n-propanol, iso-propanol, n-butanol). Rosiglitazone exhibits higher solubility in non-alcoholic solvents, whereas its solubility in alcoholic solvents is similar and relatively low. Subsequently, the correlation between the experimentally measured solubility of Rosiglitazone and the Modified Apelblat model, λh model, NRTL model, and Van’t Hoff model was examined. The Van’t Hoff equation calculations proved that the dissolution of Rosiglitazone was an endothermic process driven by entropy and characterized by non-spontaneity. The calculation of solvent polarity and Hansen solubility parameters (HSP) reveals that the primary factor influencing the solubility of Rosiglitazone is polarity in highly polar solvents, while the dominant factor is molecular volume in low-polarity solvents. Furthermore, the molecular interactions of solute and solvents were investigated by molecular simulation, including the calculation of electrostatic potential, 2D fingerprint plots and solvation free energy, which provided insights into the mechanism of Rosiglitazone dissolution behavior in different solvents.
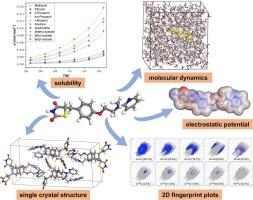
罗格列酮在十种纯溶剂中的溶解行为:模型关联、热力学计算和分子模拟
罗格列酮是一种口服抗糖尿病药物,主要用于治疗 2 型糖尿病。本文首次分析了罗格列酮的单晶结构(a = 14.0613(7) Å, b = 8.4675(4) Å, c = 29.8841(16) Å, 空间群 Pbca, Z = 8, V = 3558.1(3) Å3)。通过溶解度测量、模型关联、热力学计算和分子模拟,全面研究了罗格列酮在十种纯溶剂中的溶解情况。在 298.15 K 的溶解度顺序为丙酮、醋酸甲酯、醋酸乙酯、醋酸丁酯、乙腈、醇类溶剂(甲醇、乙醇、正丙醇、异丙醇、正丁醇)。罗格列酮在非酒精溶剂中的溶解度较高,而在酒精溶剂中的溶解度相似且相对较低。随后,研究人员考察了实验测得的罗格列酮溶解度与改良阿佩尔布拉特模型、λh 模型、NRTL 模型和 Van't Hoff 模型之间的相关性。Van't Hoff 方程计算证明,罗格列酮的溶解是一个由熵驱动的内热过程,具有非自发性的特点。溶剂极性和汉森溶解度参数(HSP)的计算表明,在高极性溶剂中,影响罗格列酮溶解度的主要因素是极性,而在低极性溶剂中,主要因素是分子体积。此外,还通过分子模拟研究了溶质和溶剂的分子相互作用,包括静电位、二维指纹图谱和溶解自由能的计算,从而深入了解了罗格列酮在不同溶剂中的溶解行为机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


