New S- and N-alkyl functionalized bis-1,2,4-Triazolyl-based derivatives as potential dual EGFRWT and EGFRT790M inhibitors: Synthesis, anti-proliferative evaluation, molecular docking study and ADMET studies
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
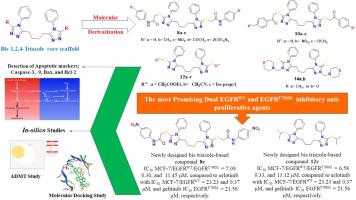
A new series of 2-(s-triazolylsulfanyl) moiety connected with N-arylacetamides 8a-e, 1-aryl ethanones 10a-c, acetate 12a, acetonitrile 12b, and 2-propane 12c were synthesized via the reaction of 5,5′-(butane-1,4-diyl)bis(4-phenyl-4H-1,2,4-triazole-3-thiol) (6) with 2‑chloro-N-arylacetamides 7a-e, phenacyl bromides 9a-c, ethyl chloroacetate 11a, chloroacetonitrile 11b, or 2-bromopropane 11c under PTC conditions. Mannich bases 14a,b were synthesized by reacting compound 6 with formaldehyde and morpholine 13a or piperidine 13b. The structure of the new products has been characterized by IR, NMR, and their elemental analyses.
These newly designed scaffolds were designed as efficient anti-proliferative EGFRWT and EGFRT790M kinase inhibitory agents. Scaffolds 8c, 10b, and 12c were established as the most potent anti-proliferative derivatives on various tested cancer cell panels with highly remarkable IC50 values compared to the reference drugs erlotinib and doxorubicin. Moreover, compounds 8c, 10b, and 12c demonstrated a favorable safety profile when tested against the normal WI-38 line, besides their exceptional selectivity as a dual kinase inhibitor, particularly targeting EGFRWT and EGFRT790M, where they were more potent than erlotinib and gefitinib, with IC50 values of (0.30 and 11.47 µM), (0.38 and 11.57 µM), (0.33 and 13.12 µM), and (0.37 and 21.56 µM), respectively. Furthermore, its apoptotic triggering capability was investigated by evaluating apoptotic markers: Caspases-3, Caspases-9, Bcl-2, Bax, and Bax/Bcl-2 ratio expression levels.
The docking outcomes of the designed scaffolds 8c, 10a, 12c, and 14b in the ATP-binding sites of both EGFRWT and EGFRT790M agreed with the in vitro results. Besides, Molecular dynamics simulations via iMODs server evaluated the stability of protein-8c complexes. Also, the in silico ADME properties’ examination of the most promising EGFR inhibitory compounds 8c, 10b, and 12c via the egg-boiled method clarified acceptable lipophilicity, GIT absorption, and blood-brain barrier penetration. So, our designed analogs, specifically 8c and 12c, own prospective anti-proliferative and dual EGFRWT and EGFRT790M kinases’ inhibitory properties, making them efficient candidates for further therapeutic amelioration in the future.
新的 S-和 N-烷基官能化双-1,2,4-三唑基衍生物作为潜在的 EGFRWT 和 EGFRT790M 双抑制剂:合成、抗增殖评估、分子对接研究和 ADMET 研究
通过 5,5′-(丁烷-1。4-二基)双(4-苯基-4H-1,2,4-三唑-3-硫醇)(6)与 2-氯-N-芳基乙酰胺 7a-e、苯基溴反应,合成了一系列新的 2-(s-三唑硫基)分子与 N-芳基乙酰胺 8a-e、1-芳基乙酮 10a-c、乙酸酯 12a、乙腈 12b 和 2-丙烷 12c、4-二基)双(4-苯基-4H-1,2,4-三唑-3-硫醇)(6)与 2-氯-N-芳基乙酰胺 7a-e、苯基溴化物 9a-c、氯乙酸乙酯 11a、氯乙腈 11b 或 2-溴丙烷 11c 在 PTC 条件下反应合成。曼尼希碱 14a,b 是通过化合物 6 与甲醛和吗啉 13a 或哌啶 13b 反应合成的。这些新设计的支架被设计为高效的抗增殖表皮生长因子受体-WT 和表皮生长因子受体-T790M 激酶抑制剂。与参考药物厄洛替尼(erlotinib)和多柔比星(doxorubicin)相比,支架 8c、10b 和 12c 是在各种测试的癌细胞面板上最有效的抗增殖衍生物,其 IC50 值非常显著。此外,化合物 8c、10b 和 12c 在针对正常 WI-38 株系的测试中表现出良好的安全性,此外,它们还具有作为双激酶抑制剂的特殊选择性,特别是针对表皮生长因子受体-WT 和表皮生长因子受体-T790M,它们比厄洛替尼和吉非替尼更有效,IC50 值分别为(0.30 和 11.47 µM)、(0.38 和 11.57 µM)、(0.33 和 13.12 µM)和(0.37 和 21.56 µM)。此外,还通过评估细胞凋亡标记物来研究其引发细胞凋亡的能力:设计的支架 8c、10a、12c 和 14b 与 EGFRWT 和 EGFRT790M 的 ATP 结合位点的对接结果与体外结果一致。此外,通过 iMODs 服务器进行的分子动力学模拟评估了蛋白质-8c 复合物的稳定性。此外,通过煮蛋法对最有前途的表皮生长因子受体抑制化合物 8c、10b 和 12c 进行的硅学 ADME 特性检查明确了可接受的亲脂性、胃肠道吸收和血脑屏障渗透。因此,我们设计的类似物,特别是 8c 和 12c,具有抗增殖和 EGFRWT 与 EGFRT790M 激酶双重抑制的前瞻性,是未来进一步改善治疗效果的有效候选化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


