Selective synthesis of dihydro- and dehydrogenated spirocyclopenta[b]indoles via acid-modulated [3+2] cycloaddition reaction
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
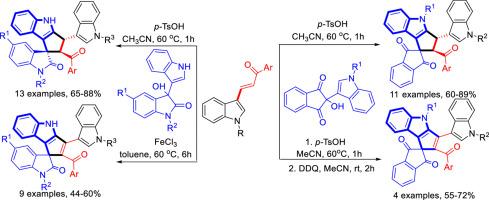
p-TsOH catalyzed [3+2] cycloaddition of 3-benzoylvinylindoles with 3-hydroxy-3-(indol-3-yl)indolin-2-ones in acetonitrile gave functionalized 3,4-dihydrospiro[cyclopenta[b]indole-1,3′-indolin]-2′-ones in good yields and with high diastereoselectivity. In the presence of FeCl3, the similar reaction of 3-benzoylvinylindoles with 3-hydroxy-3-(indol-3-yl)indolin-2-ones in toluene afforded dehydrogenated spiro[cyclopenta[b]indole-1,3′-indolin]-2′-ones in good yields. On the other hand, p-TsOH catalyzed [3+2] cycloaddition of 3-benzoylvinylindoles and 2-hydroxy-2-(indol-3-yl)-indene-1,3-diones gave 3,4-dihydrospiro[cyclopenta[b]indole-1,2′-indene]-1′,3′-diones, which could be oxidized to dehydrogenated spiro[cyclopenta[b]indole-1,2′-indene]-1′,3′-diones by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).
通过酸调节 [3+2] 环加成反应选择性合成二氢和脱氢螺环戊并[b]吲哚
p-TsOH 催化 3-苯甲酰乙烯基吲哚与 3-羟基-3-(吲哚-3-基)吲哚啉-2-酮在乙腈中发生 [3+2] 环加成反应,得到了官能化的 3,4-二氢螺[环戊并[b]吲哚-1,3′-吲哚啉]-2′-酮,产率高,非对映选择性强。在 FeCl3 存在下,3-苯甲酰基乙烯基吲哚与 3-羟基-3-(吲哚-3-基)吲哚啉-2-酮在甲苯中发生类似反应,得到脱氢的螺[环戊并[b]吲哚-1,3′-吲哚啉]-2′-酮,产率良好。另一方面,在 p-TsOH 催化下,3-苯甲酰乙烯基吲哚和 2-羟基-2-(吲哚-3-基)-茚-1,3-二酮发生 [3+2] 环加成反应,得到 3,4-二氢螺[环戊并[b]吲哚-1、2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)可将其氧化为脱氢螺[环戊并[b]吲哚-1,2′-茚]-1′,3′-二酮。)
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


