Synthesis, characterization, natural bond orbital and nonlinear optical exploration of Cu(II), Co(II) and Ni(II) complexes derived from hydrazone Schiff base ligands
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In current research work, new hydrazone ligands (HL1-HL2) and their copper(II), nickel(II), and cobalt(II) complexes were prepared and categorized by various spectroscopic (FT-IR, proton NMR, carbon NMR and UV–vis) and physical techniques. To compare the theoretical and experimental findings, density functional theory calculations were executed at the B3LYP/6-31G (d,p) level for the following parameters: molecular electrostatic potential (MEP), global reactivity parameters, frontier molecular orbital (FMO) analysis, natural bond orbital (NBO) analysis and first hyperpolarizability analysis. The FMO analysis of the synthesized compounds has shown the potential for charge transfer in the newly synthesized compounds. The GRPs observed that all the synthesized compounds exhibited increased hardness and decreased softness, suggesting reduced reactivity and enhanced stability. The NLO properties of the complexes (1–6) are shown more polarized than their corresponding hydrazone Schiff-base ligands HL1-HL2. Various parameters were studied in ADME/T analysis. Our theoretical study shows that the synthesized compounds are potential applicants for NLO applications with properties similar to those of common NLO materials used in different technological fields.
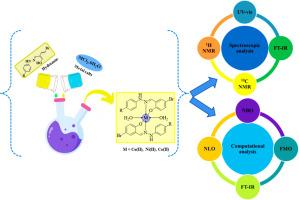
由腙席夫碱配体衍生的 Cu(II)、Co(II) 和 Ni(II) 复合物的合成、表征、自然键轨道和非线性光学探索
在当前的研究工作中,制备了新的腙配体(HL1-HL2)及其铜(II)、镍(II)和钴(II)配合物,并通过各种光谱(傅立叶变换红外光谱、质子核磁共振、碳核磁共振和紫外可见光)和物理技术对其进行了分类。为了比较理论和实验结果,在 B3LYP/6-31G (d,p) 水平上对以下参数进行了密度泛函理论计算:分子静电位 (MEP)、全局反应性参数、前沿分子轨道 (FMO) 分析、天然键轨道 (NBO) 分析和第一超极化率分析。对合成化合物的前沿分子轨道分析表明,新合成的化合物具有电荷转移的潜力。GRPs 观察发现,所有合成化合物都表现出硬度增加、柔软度降低的特点,这表明反应活性降低、稳定性增强。与相应的腙席夫碱配体 HL1-HL2 相比,复合物(1-6)的 NLO 特性更具极性。在 ADME/T 分析中研究了各种参数。我们的理论研究表明,合成的化合物具有类似于不同技术领域常用 NLO 材料的特性,是 NLO 应用的潜在应用领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


