Microwave-assisted synthesis, characterization and molecular docking of two C2-symmetric Schiff bases as fluorescent dye for silk fibroin
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
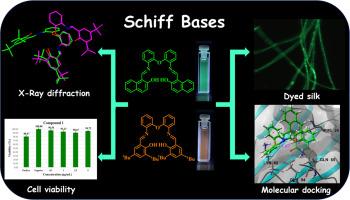
Using non-cytotoxic fluorescent materials for staining Bombyx mori silk fibroin holds great promise for tissue engineering and regenerative medicine, as it could be used as a scaffold and monitor for cell growth. Here, we report the microwave-assisted synthesis of two fluorescent Schiff bases in short reaction time and good yields. Detailed characterization of the synthesized compounds using NMR (1H, 13C NMR, and 2D NMR experiments), HRMS (DART+), UV/Vis, fluorescence spectroscopy, and X-ray diffraction studies. The Schiff base molecular structures showed a non-planar arrangement with the C2 point group. Both compounds showed low cytotoxicity on colon carcinoma cells (Caco2, ATCC![]() HTB-37) at different concentrations (1 to 5 μg/mL). Notably, Schiff bases exhibit good fluorescent staining of Bombyx mori silk fibroin by the immersion method and were analyzed by confocal microscopy. The fluorescent staining ability of the Schiff bases toward the silk fibroin was explored by molecular docking, where two predominant binding sites were identified, and H-bonding and other polar interactions were found to stabilize the binding modes of the compounds. From the remarkable findings and to the best of our knowledge, this is the first report on Schiff bases as a fluorescent dye of this biomaterial.
HTB-37) at different concentrations (1 to 5 μg/mL). Notably, Schiff bases exhibit good fluorescent staining of Bombyx mori silk fibroin by the immersion method and were analyzed by confocal microscopy. The fluorescent staining ability of the Schiff bases toward the silk fibroin was explored by molecular docking, where two predominant binding sites were identified, and H-bonding and other polar interactions were found to stabilize the binding modes of the compounds. From the remarkable findings and to the best of our knowledge, this is the first report on Schiff bases as a fluorescent dye of this biomaterial.
微波辅助合成、表征和分子对接两种用作丝纤维素荧光染料的 C2 对称席夫碱
使用无毒荧光材料染色蚕丝纤维素在组织工程和再生医学中大有可为,因为它可用作支架和细胞生长监测器。在此,我们报告了微波辅助合成两种荧光席夫碱的方法,反应时间短,产率高。利用核磁共振(1H、13C NMR 和 2D NMR 实验)、HRMS(DART+)、紫外/可见光谱、荧光光谱和 X 射线衍射研究对合成的化合物进行了详细表征。希夫碱分子结构显示出 C2 点基团的非平面排列。在不同浓度(1 至 5 μg/mL)下,这两种化合物对结肠癌细胞(Caco2、ATCCHTB-37)的细胞毒性都很低。值得注意的是,希夫碱通过浸泡法对蚕丝纤维素具有良好的荧光染色作用,并通过共聚焦显微镜进行了分析。通过分子对接研究了希夫碱对蚕丝纤维素的荧光染色能力,发现了两个主要的结合位点,并发现 H 键和其他极性相互作用稳定了化合物的结合模式。据我们所知,这是有关希夫碱作为这种生物材料的荧光染料的首次报道。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


