Theoretical and experimental insights of two surfactants’ effects on SnIn electrodeposited coatings from ethaline
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
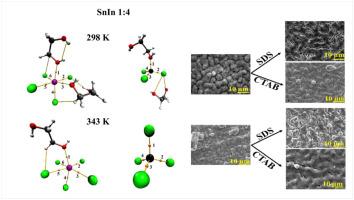
This work uses computational methodologies to investigate the behavior of Sn2+ and In3+ ions in deep eutectic solvent (DES) based on choline chloride and ethylene glycol (1ChCl:2EG, ethaline), under different conditions, such as different temperatures, ion ratios, and in the presence and absence of hexadecyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) surfactants. Furthermore, SnIn alloys were electrodeposited on Cu surfaces to analyze the effect of the investigated different conditions on the surface morphology and chemical composition of the electrodeposited coatings. Morphology and composition were analyzed using Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDS). For computational approach, twelve Molecular Dynamics (MD) simulations were conducted using the GROMACS software. After MD simulations, Quantum Theory of Atoms in Molecules (QTAIM) properties were obtained. The results presented that In3+ showed a stronger affinity for Cl− than Sn2+ but it presents weaker and less stable interactions with OA (OA = oxygen of ethylene glycol-EG), particularly at higher temperatures. Electron Density and Electron Localization Function analysis indicated that In-Cl interactions are stronger and more polarized than other interactions. The Laplacian of Electron Density suggested an intermolecular nature for all interactions. The surfactant addition altered these interactions slightly, with CTAB reducing the density of Cl− around In3+ and SDS positively interacting with In3+ at 297 K and in a Sn2+:In3+ 1:1. As SDS interacted more with In3+ ions, the electrodeposition times varied between 4965 s and 75,541 s. SEM images revealed temperature- and potential-dependent changes in film morphology, changing from globular to smooth. CTAB and SDS additions led to uniform coating in Sn2+: In3+ solutions. For the 1:1 ratio at −1.4 V, there were no significant changes (between 75 and 77 %) in the percentage of Sn deposited with the use of surfactants at 297 K. At 343 K, the surfactant-free coating exhibited 69 % Sn, CTAB facilitated the Sn deposition, increasing the percentage to 92 %. At the same time, the presence of the SDS resulted in 79 % Sn. Similar behavior was obtained for −1.0 V. For 1:4 at −1.0 V at 297 K, 35 % of Sn was observed without surfactant, 29 % with CTAB, and 33 % with SDS. At 343 K, there was an increase in Sn content for 42 %, 49 % and 60 %, indicating the positive influence of the temperature, respectively. At −1.4 V, however, there was no observed influence on the Sn percentages without surfactant. With CTAB (between 29 and 34 %) at 297 and 343 K. At these temperatures, the addition of SDS promoted a higher content of Sn, increasing to 56 % and 43 %, respectively.
两种表面活性剂对乙醇电沉积锡涂层影响的理论和实验启示
本研究利用计算方法研究了 Sn2+ 和 In3+ 离子在基于氯化胆碱和乙二醇(1ChCl:2EG, ethaline)的深共晶溶剂(DES)中,在不同温度、离子比率、存在或不存在十六烷基三甲基溴化铵(CTAB)和十二烷基硫酸钠(SDS)表面活性剂等不同条件下的行为。此外,还在铜表面电沉积了铟锡合金,以分析所研究的不同条件对电沉积涂层的表面形态和化学成分的影响。使用扫描电子显微镜(SEM)和能量色散 X 射线(EDS)分析了形态和成分。在计算方法方面,使用 GROMACS 软件进行了十二次分子动力学(MD)模拟。在 MD 模拟之后,获得了分子中原子的量子理论(QTAIM)特性。结果表明,In3+ 与 Cl- 的亲和力强于 Sn2+,但与 OA(OA = 乙二醇-EG 中的氧)的相互作用较弱且不稳定,尤其是在较高温度下。电子密度和电子定位函数分析表明,In-Cl 的相互作用比其他相互作用更强、更极化。电子密度的拉普拉斯函数表明所有相互作用都具有分子间性质。在 297 K 和 Sn2+:In3+ 1:1 的条件下,CTAB 会降低 In3+ 周围 Cl- 的密度,而 SDS 会与 In3+ 发生正向相互作用。SEM 图像显示了薄膜形态随温度和电位的变化,从球状变为光滑。在 Sn2+: In3+ 溶液中添加 CTAB 和 SDS 可形成均匀的涂层。对于 -1.4 V 下的 1:1 比例,在 297 K 下,使用表面活性剂后沉积的 Sn 百分比没有显著变化(在 75% 和 77% 之间);在 343 K 下,不含表面活性剂的镀层显示出 69% 的 Sn,CTAB 促进了 Sn 的沉积,使百分比增加到 92%。同时,SDS 的存在使锡的沉积率达到 79%。在 -1.0 V 下也有类似的表现。在 297 K 时,1:4 的电压为 -1.0 V,在不使用表面活性剂的情况下,观察到的锡含量为 35%,使用 CTAB 时为 29%,使用 SDS 时为 33%。在 343 K 时,锡含量分别增加了 42%、49% 和 60%,这表明温度具有积极影响。然而,在 -1.4 V 的电压下,没有观察到表面活性剂对锡含量的影响。在 297 K 和 343 K 的温度下,加入 CTAB(介于 29% 和 34% 之间)。在这些温度下,加入 SDS 会提高锡的含量,分别增加到 56% 和 43%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


