Functionalization of thiazolo[5,4-c]isoquinolines through Suzuki–Miyaura coupling
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The functionalization of halogenated thiazolo[5,4-c]isoquinolines (TzIQ) through the Suzuki–Miyaura reaction with arylboronic acids is reported here for the first time. Three TzIQ derivatives bearing only chlorine atoms or chlorine and bromine atoms were used in this work. The results unequivocally confirm the impact of different halogen atoms, and their positions on the TzIQ core, on the outcome of these reactions. Excellent chemoselectivity was observed for the TzIQ bearing chlorine and bromine atoms. The new TzIQ derivatives were fully characterized by NMR and MS techniques and the structure of the TzIQ 9b was also confirmed by single-crystal X-ray diffraction. The absorption and emissive properties of the new compounds indicate that some of them may be useful in applications requiring strong luminescence and large Stokes shifts.
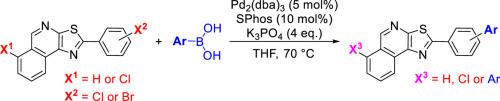
通过铃木-宫浦偶联使噻唑并[5,4-c]异喹啉官能化
本文首次报道了卤代噻唑并[5,4-c]异喹啉(TzIQ)通过与芳基硼酸的 Suzuki-Miyaura 反应进行官能化的过程。这项研究使用了三种仅含有氯原子或氯和溴原子的 TzIQ 衍生物。研究结果明确证实了不同卤素原子及其在 TzIQ 核心上的位置对这些反应结果的影响。带有氯原子和溴原子的 TzIQ 具有极佳的化学选择性。核磁共振和质谱技术对新的 TzIQ 衍生物进行了全面表征,单晶 X 射线衍射也证实了 TzIQ 9b 的结构。新化合物的吸收和发射特性表明,它们中的一些可能有助于需要强发光和大斯托克斯位移的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


