Interaction-Dependent Secondary Structure of Peptides in Biomolecular Condensates
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Biomolecular condensates provide a mechanism for compartmentalization of biomolecules in eukaryotic cells. These liquid-like condensates are formed via liquid–liquid phase separation, by a plethora of interactions, and can mediate several biological processes in healthy cells. Expansions of dipeptide repeat proteins, DPRs, in which arginine rich DPRs like poly-proline-arginine (PR), and poly-glycine-arginine (GR), partition RNA into condensates can however induce cell toxicity. Here, we use (GR)20 as a model for biological poly-GR and condense it using either excluded volume interactions with polyethylene glycol (PEG) as a crowder or direct electrostatic interactions with RNA oligomers. Using two-dimensional infrared (2D IR) spectroscopy, we observe that (GR)20 condensed through an excluded volume forms β-sheet structures, whereas (GR)20 condensed with RNA forms loops. We also investigate local hydrogen-bond dynamics in the condensate and compare the measurements with molecular dynamics simulations. Hydrogen bond lifetimes undergo a marked slowdown compared to dynamics in the dilute phase. This is representative of confined water within the percolated networks inside the condensate due to the interaction present in the condensate disrupting H-bond networks. Overall, our results show that both protein structure and dynamics are inherently dependent on the type of interactions that stabilize the condensates.
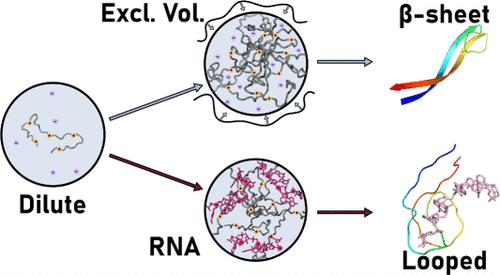
生物分子凝聚体中肽的二级结构与相互作用有关
生物分子凝聚物为真核细胞中生物分子的分隔提供了一种机制。这些液态凝集物通过液-液相分离和大量的相互作用形成,可以介导健康细胞中的多个生物过程。然而,二肽重复蛋白(DPRs)的扩增(其中富含精氨酸的二肽重复蛋白如多脯氨酸-精氨酸(PR)和多甘氨酸-精氨酸(GR)将 RNA 分隔成凝聚体)会诱发细胞毒性。在这里,我们使用 (GR)20 作为生物聚-GR 的模型,并利用聚乙二醇 (PEG) 作为挤出剂的排除体积相互作用或与 RNA 低聚物的直接静电相互作用将其凝结。利用二维红外光谱(2D IR),我们观察到通过排除体积缩合的 (GR)20 形成了 β 片状结构,而与 RNA 缩合的 (GR)20 则形成了环状结构。我们还研究了冷凝物中的局部氢键动力学,并将测量结果与分子动力学模拟进行了比较。与稀释相中的动力学相比,氢键寿命明显减慢。这是由于冷凝液中存在的相互作用破坏了氢键网络,在冷凝液内部的渗透网络中产生了封闭水。总之,我们的研究结果表明,蛋白质结构和动力学本质上都依赖于稳定凝聚态的相互作用类型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


