Cyclodextrin-Grafted Poly(vanillin) Antimicrobial Bio-Nanohoops via “Graft From” RAFT and Supramolecular Host–Guest Chemistry
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Microbial infections have been recognized as one of the most serious threats to healthcare and agriculture production, and it is still a great challenge to explore antimicrobial biomaterials with supramolecular self-assembling systems. To address this challenge, novel bionanohoops were fabricated via “graft from” reversible addition–fragmentation chain transfer (RAFT) and supramolecular host–guest chemistry. Admittedly, controllable grafting of vanillin-derived homopolymer (PVMAx) from β-cyclodextrin (β-CD) to synthesize β-cyclodextrin-grafted poly(vanillin methacrylate) (β-CD-g-PVMAx, x = 5, 35, 103) was calculated from 1H NMR integral area, and 2D NOESY demonstrated that the primary structured linear homopolymer chains (β-CD-g-PVMA5) were linked to each other by host–guest interactions. Additionally, GPC results illustrated that the secondary structured nanohoops ([β-CD-g-PVMA5]y, y = 38 or 364) were self-assembled in situ from β-CD-g-PVMA5 through supramolecular host–guest chemistry. Compared with stacking nanorods, nanohoops not only exhibited excellent antibacterial and antifungal activities but also presented good biocompatibility and better paint adhesion. Overall, we provided a valuable strategy that constructs antimicrobial bionanohoops by combining “graft from” RAFT and supramolecular host–guest chemistry for addressing microbial infections.
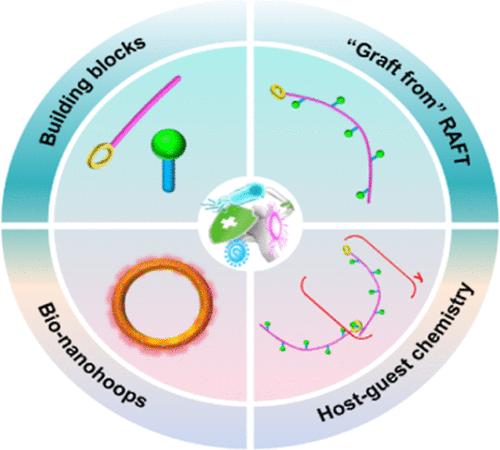
通过 "接枝 "RAFT 和超分子主客体化学实现环糊精接枝聚(香兰素)抗菌生物纳米环
微生物感染已被公认为是对医疗保健和农业生产最严重的威胁之一,而利用超分子自组装系统探索抗菌生物材料仍是一项巨大的挑战。为了应对这一挑战,我们通过 "嫁接 "可逆加成-断裂链转移(RAFT)和超分子宿主-宿主化学制备了新型仿生环。根据 1H NMR 积分面积计算出香兰素衍生均聚物(PVMAx)与β-环糊精(β-CD)的可控接枝,从而合成了β-环糊精接枝聚(甲基丙烯酸香兰素)(β-CD-g-PVMAx,x = 5、35、103)。此外,GPC 结果表明,次结构纳米环([β-CD-g-PVMA5]y,y = 38 或 364)是由 β-CD-g-PVMA5 通过超分子主-客化学作用在原位自组装而成的。与堆叠纳米棒相比,纳米环不仅具有出色的抗菌和抗真菌活性,还具有良好的生物相容性和更好的涂料附着力。总之,我们提供了一种有价值的策略,即通过结合 "嫁接 "RAFT 和超分子主宾化学来构建抗菌仿生纳米环,从而解决微生物感染问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


