Dural Tregs driven by astrocytic IL-33 mitigate depression through the EGFR signals in mPFC neurons
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
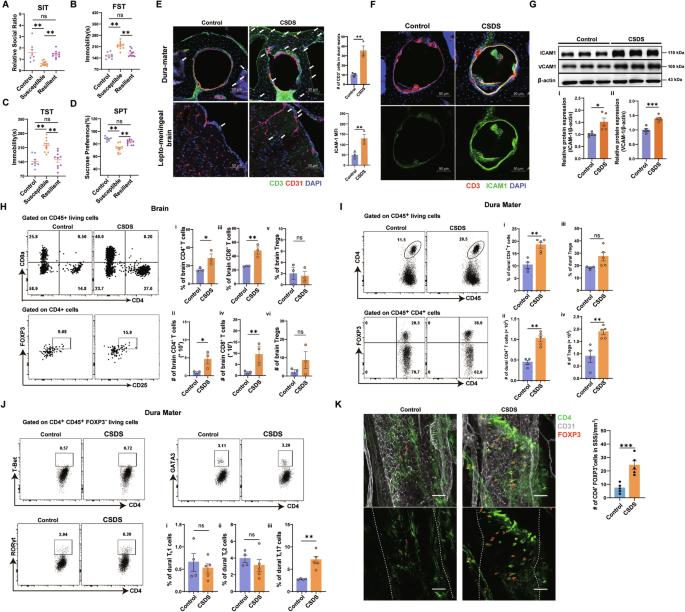
The dura sinus-resident immune cells can influence the process of central neural system (CNS) diseases by communicating with central nerve cells. In clinical, Tregs are also frequently impaired in depression. However, the significance of this relationship remains unknown. In the present study, we found a significant increase in dural Treg populations in mouse models of depression, whereas depleting them by neutralizing antibodies injection could exacerbate depressive phenotypes. Through RNA sequencing, we identified that the antidepressant effects of dural Tregs are at least in part through the production of amphiregulin, increasing the expression of its receptor EGFR in medial prefrontal cortex (mPFC) pyramidal neurons. Furthermore, dural Tregs expressed high levels of ST2, and their expansion in depressed mice depended on astrocyte-derived IL33 secretion. Our study shows that dural Treg signaling can be enhanced by treatment with fluoxetine, highlighting that dural Tregs can be utilized as a potential target cell in major depressive disorder (MDD).
由星形胶质细胞 IL-33 驱动的硬脑膜集落通过表皮生长因子受体信号减轻 mPFC 神经元的抑郁状况
硬脑膜窦驻留的免疫细胞可通过与中枢神经细胞的交流影响中枢神经系统(CNS)疾病的进程。在临床上,抑郁症患者的Tregs也经常受损。然而,这种关系的重要性仍然未知。在本研究中,我们发现在抑郁症小鼠模型中,硬脑膜Treg群显著增加,而通过注射中和抗体来消耗它们会加重抑郁症表型。通过RNA测序,我们发现硬脑膜Tregs的抗抑郁作用至少部分是通过产生表皮生长因子,增加其受体表皮生长因子受体在内侧前额叶皮层(mPFC)锥体神经元中的表达。此外,硬脑膜Tregs表达高水平的ST2,它们在抑郁小鼠体内的扩增依赖于星形胶质细胞衍生的IL33分泌。我们的研究表明,用氟西汀治疗可增强硬脑膜Treg信号转导,这突出表明硬脑膜Tregs可被用作重度抑郁障碍(MDD)的潜在靶细胞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


