Crown-like Biodegradable Lipids Enable Lung-Selective mRNA Delivery and Dual-Modal Tumor Imaging In Vivo
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Systemic mRNA delivery to specific cell types remains a great challenge. We herein report a new class of crown-like biodegradable ionizable lipids (CBILs) for predictable lung-selective mRNA delivery by leveraging the metal coordination chemistry. Each CBIL contains an impressive crown-like amino core that coordinates with various metal ions such as Zn2+ and further regulates the in vivo organ-targeting behavior of lipid nanoparticles (LNPs). The representative CBIL (Zn-9C-SCC-10)-formulated LNPs could exclusively deliver mRNA to the lung after systemic administration. Notably, following intravenous administration of 0.2 mg kg–1 Cre mRNA, Zn-9C-SCC-10 LNPs enabled the highly efficient gene editing of all lung epithelial and endothelial cells up to 43 and 61%, respectively, outperforming the current state-of-the-art LNPs in lung epithelial cell delivery. Moreover, compared to DLin-MC3-DMA LNPs with the addition of cationic lipid (DOTAP), our approach yielded a 44.6-fold enhancement in pulmonary mRNA expression and significantly improved biosafety in vivo. Taking advantage of paramagnetic gadolinium ion, Gd-12C-SCC-10 LNPs allowed the potent mRNA delivery to cancer cells and successfully illuminated lung tumors by magnetic and bioluminescent dual-mode imaging, facilitating the early discovery and diagnosis of lung cancer. This work will open a new avenue to rationally design predictable LNPs, as well as address the major challenges of mRNA delivery to specific cells in the lung tissues for treating a wide variety of diseases.
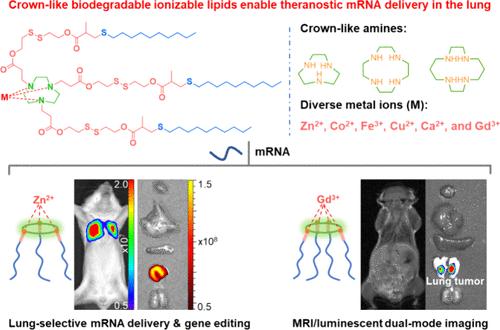
皇冠样生物可降解脂质实现肺选择性 mRNA 运送和体内双模式肿瘤成像
将 mRNA 系统性地传递到特定细胞类型仍然是一项巨大的挑战。我们在此报告了一类新的冠状生物可降解可电离脂质(CBILs),通过利用金属配位化学可预测肺选择性 mRNA 递送。每种 CBIL 都含有一个令人印象深刻的冠状氨基核心,可与 Zn2+ 等多种金属离子配位,并进一步调节脂质纳米颗粒(LNPs)的体内器官靶向行为。具有代表性的 CBIL(Zn-9C-SCC-10)制备的 LNPs 可在全身给药后将 mRNA 独家输送到肺部。值得注意的是,静脉注射 0.2 mg kg-1 Cre mRNA 后,Zn-9C-SCC-10 LNPs 对所有肺上皮细胞和内皮细胞的高效基因编辑率分别高达 43% 和 61%,在肺上皮细胞递送方面优于目前最先进的 LNPs。此外,与添加阳离子脂质(DOTAP)的DLin-MC3-DMA LNPs相比,我们的方法在肺mRNA表达方面提高了44.6倍,并显著提高了体内生物安全性。利用顺磁性钆离子的优势,Gd-12C-SCC-10 LNPs 可将 mRNA 强效递送至癌细胞,并通过磁性和生物发光双模式成像成功照亮肺部肿瘤,有助于肺癌的早期发现和诊断。这项工作将为合理设计可预测的 LNPs 开辟一条新途径,并解决将 mRNA 运送到肺组织中特定细胞以治疗多种疾病的重大难题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


