Isocyanide Ligation Enables Electrochemical Ammonia Formation in a Synthetic Cycle for N2 Fixation
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
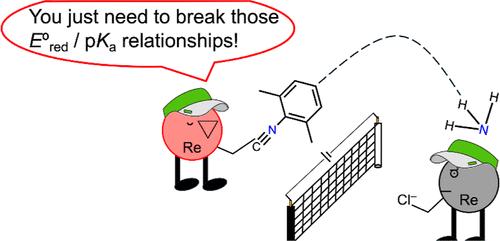
Transition-metal-mediated splitting of N2 to form metal nitride complexes could constitute a key step in electrocatalytic nitrogen fixation, if these nitrides can be electrochemically reduced to ammonia under mild conditions. The envisioned nitrogen fixation cycle involves several steps: N2 binding to form a dinuclear end-on bridging complex with appropriate electronic structure to cleave the N2 bridge followed by proton/electron transfer to release ammonia and bind another molecule of N2. The nitride reduction and N2 splitting steps in this cycle have differing electronic demands that a catalyst must satisfy. Rhenium systems have had limited success in meeting these demands, and studying them offers an opportunity to learn strategies for modulating reactivity. Here, we report a rhenium system in which the pincer supporting ligand is supplemented by an isocyanide ligand that can accept electron density, facilitating reduction and enabling the protonation/reduction of the nitride to ammonia under mild electrochemical conditions. The incorporation of isocyanide raises the N–H bond dissociation free energy of the first N–H bond by 10 kcal/mol, breaking the usual compensation between pKa and redox potential; this is attributed to the separation of the protonation site (nitride) and the reduction site (delocalized between Re and isocyanide). Ammonia evolution is accompanied by formation of a terminal N2 complex, which can be oxidized to yield bridging N2 complexes including a rare mixed-valent complex. These rhenium species define the steps in a synthetic cycle that converts N2 to NH3 through an electrochemical N2 splitting pathway, and show the utility of a second, tunable supporting ligand for enhancing nitride reactivity.
异氰酸酯连接使电化学氨在氮固定合成循环中形成
如果这些氮化物能在温和的条件下通过电化学还原成氨,那么过渡金属介导的 N2 分离形成金属氮化物复合物可能会成为电催化固氮的一个关键步骤。设想中的固氮循环包括几个步骤:N2 结合形成具有适当电子结构的双核端对桥复合物,从而裂解 N2 桥,然后进行质子/电子转移以释放氨并结合另一个 N2 分子。这一循环中的氮化物还原和 N2 裂解步骤对催化剂的电子要求各不相同,催化剂必须满足这些要求。铼系统在满足这些要求方面取得的成功有限,而对它们的研究则为我们提供了学习调节反应活性策略的机会。在这里,我们报告了一种铼体系,其中的钳形支撑配体由异氰酸配体补充,该配体可以接受电子密度,促进还原,并使氮化物在温和的电化学条件下质子化/还原成氨。异氰化物的加入使第一个 N-H 键的 N-H 键解离自由能提高了 10 kcal/mol,打破了 pKa 和氧化还原电位之间的通常补偿;这归因于质子化位点(氮化物)和还原位点(Re 和异氰化物之间的脱位)的分离。氨的演化伴随着末端 N2 复合物的形成,该复合物可被氧化生成桥接 N2 复合物,包括一种罕见的混合价复合物。这些铼物种确定了通过电化学 N2 分离途径将 N2 转化为 NH3 的合成循环步骤,并显示了第二种可调支持配体在提高氮化物反应性方面的效用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


