Photocatalyzed Selective α-Monoalkylation of Aqueous Acetaldehyde for Synthesis of α,β-Unsubstituted 1,4-Ketoaldehydes
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The direct utilization of aqueous acetaldehyde as an industrially important aqueous chemical has been hampered in organic synthesis due to the high reactivity of acetaldehyde. Herein, the first metal-free photocatalyzed selective α-monoalkylation of commercially available and safe to handle aqueous acetaldehyde (40 wt %) with inexpensive α-chloro/bromoacetophenones has been developed using 0.5 mol % 4CzIPN as an organic photosensitizer, which provides easy access to high-added-value α,β-unsubstituted 1,4-ketoaldehydes. The reaction features a broad substrate scope, high functional group tolerance, gram-scalability, operational simplicity, and mild reaction conditions. Moreover, the synthetic applications are demonstrated by directly using aqueous solutions of acetaldehyde as synthetically useful a C2 synthon for the modular and expeditious synthesis of various biologically active γ-aminoketones through the reductive amination of the α,β-unsubstituted 1,4-ketoaldehyde products with cyclic secondary amines, as well as the concise gram-scale total synthesis of pentabromo- and pentachloropseudilins.
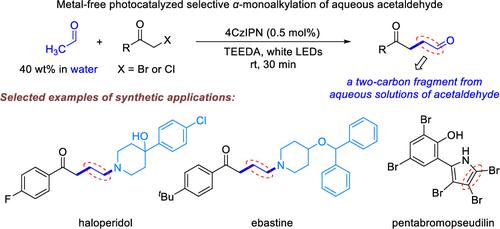
光催化乙醛水溶液的选择性 α-单烷基化合成 α、β-未取代的 1,4-酮醛
由于乙醛的高反应性,水基乙醛作为一种重要的工业水基化学品,在有机合成中的直接利用一直受到阻碍。在此,我们以 0.5 mol % 的 4CzIPN 作为有机光敏剂,首次开发了无金属光催化选择性α-单烷基化技术,将市售且可安全处理的乙醛(40 wt %)与廉价的α-氯/溴苯乙酮进行α-单烷基化反应,从而轻松获得高附加值的α,β-未取代的 1,4-酮醛。该反应具有底物范围广、官能团耐受性高、可克级放大、操作简单和反应条件温和等特点。此外,通过α,β-未取代的 1,4-酮醛产物与环状仲胺的还原胺化反应,直接使用乙醛水溶液作为有用的 C2 合物,模块化地快速合成各种具有生物活性的γ-氨基酮,以及以克为单位简便地全合成五溴和五氯伪丝氨酸,证明了该合成方法的应用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


