Geometric and Symmetric Adaptations of Crown Ether upon Its Coordination to Alkali Metal Cations on the Surface
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Crown ethers (CRs), known for their selective affinity toward alkali metal cations, play a crucial role in supramolecular chemistry. In this study, scanning tunneling microscopy and qPlus-atomic force microscopy are employed to visualize 18-crown-6 (18C6) complexes with various alkali metal cations (Li+, K+, and Cs+) on Cu(110) at a submolecular level. These visualizations are supplemented by theoretical calculations and simulations that reveal the geometric and symmetrical adaptations occurring upon the cation accommodations. It is observed that upon its coordination to the K+ cation, the 18C6 molecule transforms its crown ring into a regular triangular feature in D3d symmetry with a binding energy of 1.21 eV. In contrast, the 18C6 molecule maintains a similar triangular feature in C3v symmetry upon its coordination to the Cs+ cation, but the formed complex loses its planar symmetry, and the Cs+ cation is about 110 pm vertically off the central oxygen plane of the 18C6 molecule, resulting in a reduced binding energy of 0.57 eV. However, the most striking change occurs upon its coordination to the Li+ cation; the 18C6 molecule depicts two configurations, an elongated triangle and a distorted one, in C1 and Cs symmetry, with significantly lower binding energies of 0.08 and 0.31 eV, respectively. These symmetrical variations in the coordination complexes highlight the optimal compatibility of the 18C6 molecule with the K+ cation. Our experimental study provides a model case with atomic insights into the host–guest recognition mechanisms of the formed alkali cation–CR complexes.
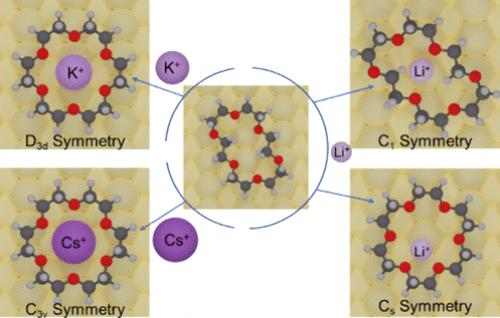
冠醚与碱金属阳离子表面配位时的几何和对称嬗变
冠醚(CR)因其对碱金属阳离子的选择性亲和力而闻名,在超分子化学中发挥着至关重要的作用。本研究采用扫描隧道显微镜和 qPlus 原子力显微镜,在亚分子水平上观察了 18-冠醚-6(18C6)与各种碱金属阳离子(Li+、K+ 和 Cs+)在 Cu(110)上的配合物。理论计算和模拟对这些可视化效果进行了补充,揭示了阳离子配位时发生的几何和对称性变化。据观察,在与 K+ 阳离子配位时,18C6 分子将其冠环转变为 D3d 对称的规则三角形特征,结合能为 1.21 eV。相比之下,18C6 分子在与 Cs+ 阳离子配位时保持了类似的 C3v 对称三角形特征,但形成的复合物失去了平面对称性,Cs+ 阳离子垂直于 18C6 分子中心氧平面约 110 pm,导致结合能降低了 0.57 eV。然而,最显著的变化发生在 18C6 与 Li+ 阳离子配位时;18C6 分子呈现出 C1 和 Cs 对称的两种构型,一种是拉长的三角形,另一种是扭曲的三角形,结合能分别显著降低到 0.08 和 0.31 eV。配位复合物的这些对称变化凸显了 18C6 分子与 K+ 阳离子的最佳相容性。我们的实验研究为所形成的碱阳离子-CR 配合物的主客体识别机制提供了一个原子洞察模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


