Picosecond Lifetimes of Hydrogen Bonds in the Halide Perovskite CH3NH3PbBr3
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The structures and properties of organic–inorganic perovskites are influenced by the hydrogen bonding between the organic cations and the inorganic octahedral networks. This study explores the dynamics of hydrogen bonds in CH3NH3PbBr3 across a temperature range from 70 to 350 K, using molecular dynamics simulations with machine-learning force fields. The results indicate that the lifetime of hydrogen bonds decreases with increasing temperature from 7.6 ps (70 K) to 0.16 ps (350 K), exhibiting Arrhenius-type behavior. The geometric conditions for hydrogen bonding, which include bond lengths and angles, maintain consistency across the full temperature range. The relevance of hydrogen bonds for the vibrational states of the material is also evidenced through a detailed analysis of the vibrational power spectra, demonstrating their significant effect on the physical properties for this class of perovskites.
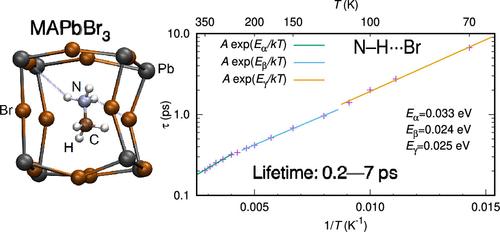
卤化物包晶 CH3NH3PbBr3 中氢键的皮秒寿命
有机无机包晶的结构和性质受到有机阳离子和无机八面体网络之间氢键的影响。本研究利用机器学习力场进行分子动力学模拟,探讨了 CH3NH3PbBr3 中氢键在 70 至 350 K 温度范围内的动态变化。结果表明,氢键的寿命随着温度的升高而减小,从 7.6 ps(70 K)减小到 0.16 ps(350 K),表现出阿伦尼乌斯型行为。氢键的几何条件(包括键长和角度)在整个温度范围内保持一致。通过对振动功率谱的详细分析,氢键与材料振动状态的相关性也得到了证明,表明氢键对这类过氧化物晶石的物理性质具有重要影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


