Next-generation aluminum adjuvants: Immunomodulatory layered double hydroxide NanoAlum reengineered from first-line drugs
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Aluminum adjuvants (Alum), approved by the US Food and Drug Administration, have been extensively used in vaccines containing recombinant antigens, subunits of pathogens, or toxins for almost a century. While Alums typically elicit strong humoral immune responses, their ability to induce cellular and mucosal immunity is limited. As an alternative, layered double hydroxide (LDH), a widely used antacid, has emerged as a novel class of potent nano-aluminum adjuvants (NanoAlum), demonstrating advantageous physicochemical properties, biocompatibility and adjuvanticity in both humoral and cellular immune responses. In this review, we summarize and compare the advantages and disadvantages of Alum and NanoAlum in these properties and their performance as adjuvants. Moreover, we propose the key features for ideal adjuvants and demonstrate that LDH NanoAlum is a promising candidate by summarizing its current progress in immunotherapeutic cancer treatments. Finally, we conclude the review by offering our integrated perspectives about the remaining challenges and future directions for NanoAlum's application in preclinical/clinical settings.
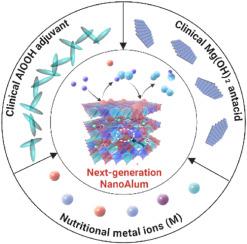
新一代铝佐剂:根据一线药物重新设计的免疫调节层状双氢氧化物纳米铝
美国食品和药物管理局批准的铝佐剂(明矾)被广泛用于含有重组抗原、病原体亚单位或毒素的疫苗中已近一个世纪。虽然明矾通常能引起强烈的体液免疫反应,但其诱导细胞和粘膜免疫的能力有限。作为一种替代品,被广泛使用的抗酸剂层状双氢氧化物(LDH)已成为一类新型的强效纳米铝佐剂(NanoAlum),在体液免疫和细胞免疫反应中均表现出良好的理化特性、生物相容性和佐剂性。在本综述中,我们总结并比较了明矾和纳米铝在这些特性方面的优缺点及其作为佐剂的性能。此外,我们还提出了理想佐剂的关键特征,并通过总结 LDH 纳米矾目前在癌症免疫治疗方面的进展,证明它是一种很有前途的候选物质。最后,我们对纳米铝在临床前/临床环境中的应用所面临的挑战和未来方向提出了自己的综合观点,从而结束了这篇综述。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


