Adipose ADM2 ameliorates NAFLD via promotion of ceramide catabolism
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The adipose tissue of mammals represents an important energy-storing and endocrine organ, and its dysfunction is relevant to the onset of several health problems, including non-alcoholic fatty liver disease (NAFLD). However, whether treatments targeting adipose dysfunction could alleviate NAFLD has not been well-studied. Adrenomedullin 2 (ADM2), belonging to the CGRP superfamily, is a protective peptide that has been shown to inhibit adipose dysfunction. To investigate the adipose tissue-specific effects of ADM2 on NAFLD, adipose-specific ADM2-overexpressing transgenic (aADM2-tg) mice were developed. When fed a high-fat diet, aADM2-tg mice displayed decreased hepatic triglyceride accumulation compared to wild-type mice, which was attributable to the inhibition of hepatic de novo lipogenesis. Results from lipidomics studies showed that ADM2 decreased ceramide levels in adipocytes through the upregulation of ACER2, which catalyzes ceramide catabolism. Mechanically, activation of adipocyte HIF2α was required for ADM2 to promote ACER2-dependent adipose ceramide catabolism as well as to decrease hepatic lipid accumulation. This study highlights the role of ADM2 and adipose-derived ceramide in NAFLD and suggests that its therapeutic targeting could alleviate disease symptoms.
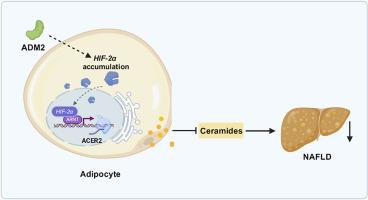
脂肪 ADM2 通过促进神经酰胺分解代谢改善非酒精性脂肪肝
哺乳动物的脂肪组织是重要的能量储存和内分泌器官,其功能障碍与包括非酒精性脂肪肝(NAFLD)在内的多种健康问题的发生有关。然而,针对脂肪功能障碍的治疗是否能缓解非酒精性脂肪肝,目前还没有很好的研究。肾上腺髓质素2(ADM2)属于CGRP超家族,是一种保护性多肽,已被证明可抑制脂肪功能障碍。为了研究ADM2对非酒精性脂肪肝的脂肪组织特异性影响,我们培育了脂肪组织特异性ADM2-表达转基因小鼠(aADM2-tg)。与野生型小鼠相比,aADM2-tg 小鼠在喂食高脂饮食时,肝脏甘油三酯的积累有所减少,这是由于肝脏新脂肪生成受到了抑制。脂质组学研究结果表明,ADM2 通过上调催化神经酰胺分解的 ACER2,降低了脂肪细胞中的神经酰胺水平。从机制上讲,ADM2 需要激活脂肪细胞 HIF2α,才能促进 ACER2 依赖性脂肪神经酰胺分解以及减少肝脏脂质积累。这项研究强调了ADM2和脂肪源性神经酰胺在非酒精性脂肪肝中的作用,并表明针对其进行治疗可减轻疾病症状。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


