Colon-specific controlled release of oral liposomes for enhanced chemo-immunotherapy against colorectal cancer
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
A colon-specific drug delivery system has great potential for the oral administration of colorectal cancer. However, the uncontrollable in vivo fate of liposomes makes their effectiveness for colonic location, and intratumoral accumulation remains unsatisfactory. Here, an oral colon-specific drug delivery system (CBS-CS@Lipo/Oxp/MTZ) was constructed by covalently conjugating Clostridium butyricum spores (CBS) with drugs loaded chitosan (CS)-coated liposomes, where the model chemotherapy drug oxaliplatin (Oxp) and anti-anaerobic bacteria agent metronidazole (MTZ) were loaded. Following oral administration, CBS germinated into Clostridium butyricum (CB) and colonized in the colon. Combined with colonic specifically β-glucosidase responsive degrading of CS, dual colon-specific release of liposomes was achieved. And the accumulation of liposomes at the CRC site furtherly increased by 2.68-fold. Simultaneously, the released liposomes penetrated deep tumor tissue via the permeation enhancement effect of CS to kill localized intratumoral bacteria. Collaborating with blocking the translocation of intestinal pathogenic bacteria from lumen to tumor with the gut microbiota modulation of CB, the intratumoral pathogenic bacteria were eliminated fundamentally, blocking their recruitment to immunosuppressive cells. Furtherly, synchronized with lipopolysaccharide (LPS) released from MTZ-induced dead Fusobacterium nucleatum and the tumor-associated antigens produced by Oxp-caused immunogenic dead cells, they jointly enhanced tumor infiltration of CD8+ T cells and reactivated robust antitumor immunity.
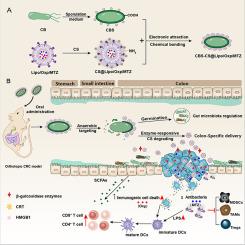
结肠特异性口服脂质体控释用于增强结直肠癌化疗免疫疗法
结肠特异性给药系统在结肠直肠癌的口服给药方面具有巨大潜力。然而,由于脂质体在体内的去向不可控,其结肠定位和瘤内蓄积的效果仍不理想。本文通过将丁酸梭菌孢子(CBS)与载药壳聚糖(CS)包裹的脂质体共价结合,构建了一种口服结肠特异性给药系统(CBS-CS@Lipo/Oxp/MTZ),其中载入了模型化疗药物奥沙利铂(Oxp)和抗厌氧菌药物甲硝唑(MTZ)。口服后,CBS 会发芽成为丁酸梭菌(CB)并在结肠中定植。结合结肠特异性β-葡萄糖苷酶对 CS 的降解反应,实现了脂质体的双重结肠特异性释放。脂质体在结肠癌部位的积累进一步增加了 2.68 倍。同时,释放的脂质体通过 CS 的渗透增强效应渗透到肿瘤深层组织,杀灭局部瘤内细菌。通过CB的肠道微生物群调节作用阻断肠道致病菌从腔隙向肿瘤的转运,从根本上清除了瘤内致病菌,阻断了它们向免疫抑制细胞的募集。此外,与 MTZ 诱导的核酸镰刀菌死亡释放的脂多糖(LPS)和 Oxp 引起的免疫原性死亡细胞产生的肿瘤相关抗原同步,它们共同增强了 CD8+ T 细胞的肿瘤浸润,重新激活了强大的抗肿瘤免疫力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


