Human mitochondrial peroxiredoxin Prdx3 is dually localized in the intermembrane space and matrix subcompartments
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Peroxiredoxin 3 (Prdx3) is the major sink for H2O2 and other hydroperoxides within mitochondria, yet the mechanisms guiding the import of its cytosolic precursor into mitochondrial sub-compartments remain elusive. Prdx3 is synthesized in the cytosol as a precursor with an N-terminal cleavable presequence, which is frequently proposed to target the protein exclusively to the mitochondrial matrix. Here, we present a comprehensive analysis of the human Prdx3 biogenesis, using highly purified mitochondria from HEK293T cells. Subfractionation and probing for specific mitochondrial markers confirmed Prdx3 localization in the matrix, while unexpectedly revealed its presence in the mitochondrial intermembrane space (IMS). Both matrix and IMS isoforms were found to be soluble proteins, as demonstrated by alkaline carbonate extraction. By combining in silico analysis, in organello import assays and heterologous expression in yeast, we found that Prdx3 undergoes sequential proteolytic processing steps by mitochondrial processing peptidase (MPP) and mitochondrial intermediate peptidase (MIP) during its import into the matrix. Additionally, heterologous expression of Prdx3 in yeast revealed that its sorting to the IMS is dependent on the inner membrane peptidase (IMP) complex. Collectively, these findings uncover a complex submitochondrial distribution of Prdx3, supporting its multifaceted role in mitochondrial H2O2 metabolism.
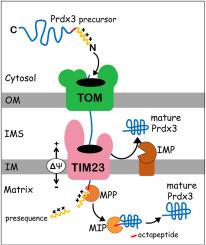
人类线粒体过氧化物酶 Prdx3 在膜间隙和基质亚区双重定位
过氧化物歧化酶 3(Prdx3)是线粒体内 H2O2 和其他氢过氧化物的主要来源,但其细胞前体导入线粒体亚区室的机制仍不明确。Prdx3 是在细胞质中合成的前体,其 N 端具有可裂解的前序,因此经常被认为是将该蛋白质专门靶向线粒体基质的原因。在这里,我们利用高度纯化的 HEK293T 细胞线粒体,对人类 Prdx3 的生物发生进行了全面分析。亚分馏和特定线粒体标记物的探测证实了 Prdx3 在基质中的定位,同时意外发现了它在线粒体膜间隙(IMS)中的存在。碱性碳酸盐提取法证明,基质和 IMS 同工酶都是可溶性蛋白。通过结合硅学分析、器官内导入试验和酵母中的异源表达,我们发现 Prdx3 在导入基质的过程中会经历线粒体加工肽酶(MPP)和线粒体中间肽酶(MIP)的连续蛋白水解处理步骤。此外,在酵母中异源表达 Prdx3 发现,其向 IMS 的分选依赖于内膜肽酶(IMP)复合物。总之,这些发现揭示了Prdx3在线粒体H2O2代谢中的多方面作用,证明了Prdx3在线粒体中的复杂的服从软骨分布。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


