A phosphoglycerate mutase 1 allosteric inhibitor restrains TAM-mediated colon cancer progression
IF 14.7
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Colorectal cancer (CRC) is a prevalent malignant tumor often leading to liver metastasis and mortality. Despite some success with PD-1/PD-L1 immunotherapy, the response rate for colon cancer patients remains relatively low. This is closely related to the immunosuppressive tumor microenvironment mediated by tumor-associated macrophages (TAMs). Our previous work identified that a phosphoglycerate mutase 1 (PGAM1) allosteric inhibitor, HKB99, exerts a range of anti-tumor activities in lung cancer. Here, we found that upregulation of PGAM1 correlates with increased levels of M2-like tumor-associated macrophages (TAMs) in human colon cancer samples, particularly in liver metastatic tissues. HKB99 suppressed tumor growth and metastasis in cell culture and syngeneic tumor models. M2-polarization, induced by colon cancer cell co-culture, was reversed by HKB99. Conversely, the increased migration of colon cancer cells by M2-TAMs was remarkably restrained by HKB99. Notably, a decrease in TAM infiltration was required for the HKB99-mediated anti-tumor effect, along with an increase in CD8+ T cell infiltration. Moreover, HKB99 improved the efficacy of anti-PD-1 treatment in syngeneic tumors. Overall, this study highlights HKB99's inhibitory activity in TAM-mediated colon cancer progression. Targeting PGAM1 could lead to novel therapeutic strategies and enhance the effectiveness of existing immunotherapies for colon cancer.
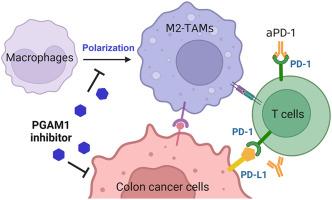
一种磷酸甘油酸突变酶 1 异源抑制剂可抑制 TAM 介导的结肠癌进展
结肠直肠癌(CRC)是一种常见的恶性肿瘤,通常会导致肝转移和死亡。尽管 PD-1/PD-L1 免疫疗法取得了一些成功,但结肠癌患者的应答率仍然相对较低。这与肿瘤相关巨噬细胞(TAMs)介导的免疫抑制性肿瘤微环境密切相关。我们之前的研究发现,磷酸甘油酸突变酶 1(PGAM1)异位抑制剂 HKB99 可在肺癌中发挥一系列抗肿瘤活性。在这里,我们发现 PGAM1 的上调与人类结肠癌样本中 M2 样肿瘤相关巨噬细胞(TAMs)水平的增加有关,尤其是在肝转移组织中。HKB99 可抑制细胞培养和合成肿瘤模型中的肿瘤生长和转移。HKB99 逆转了结肠癌细胞共培养诱导的 M2 极化。相反,HKB99 能显著抑制 M2-TAMs 对结肠癌细胞的迁移。值得注意的是,HKB99 介导的抗肿瘤效应需要 TAM 浸润的减少以及 CD8+ T 细胞浸润的增加。此外,HKB99 还提高了抗 PD-1 治疗合成肿瘤的疗效。总之,这项研究强调了 HKB99 在 TAM 介导的结肠癌进展中的抑制活性。以 PGAM1 为靶点可以开发新的治疗策略,提高现有结肠癌免疫疗法的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


