Chronic alcohol consumption exacerbates ischemia-associated skeletal muscle mitochondrial dysfunction in a murine model of peripheral artery disease
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-11-23
DOI:10.1016/j.bbadis.2024.167584
引用次数: 0
Abstract
Purpose
Peripheral artery disease (PAD) causes ischemic mitochondriopathy-associated muscle damage, amplifying patient disability and mortality. Although alcohol and a high-fat diet enhance PAD predisposition and severity, their impact on PAD myopathy is unclear. Using our murine model of PAD, we investigated the combined effect of chronic alcohol and fat consumption on intramuscular oxidative stress and mitochondrial content, function, and quality control. The potential relationship between intramuscular aldehyde dehydrogenase 2 (ALDH2) content, oxidative stress and mitochondriopathy was also explored.
Methods
Twenty-four male, 24 female, 8-month-old C57BL/6 J mice received high-fat-sucrose (HFS) or low-fat-sucrose (LFS) diets for 16-weeks, followed by either 20 % ethanol (EtOH) supplemented in the drinking water or continued water access for another 12-weeks (n = 12 mice/4 groups). The left femoral artery was ligated to induce hindlimb ischemia (HLI), and mice 4-weeks post-ligation were euthanized.
Results
Chronic HLI was associated with an ischemic muscle mitochondriopathy, which was exacerbated by concurrent HFS and EtOH feeding. Intramuscular ALDH2 was also reduced in mice consuming HFS + EtOH, particularly in the ischemic limb, but increased in their LFS + EtOH-consuming counterparts. Moreover, reduced ALDH2 was strongly correlated with markers of oxidative stress and mitochondrial dysfunction.
Conclusions
ALDH2 could be a promising therapeutic target to optimize intramuscular mitochondrial function in PAD patients, particularly those who habitually consume a diet high in fat and alcohol.
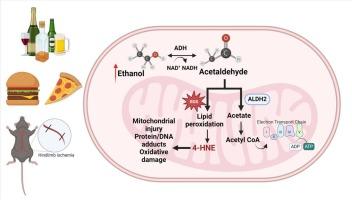
在小鼠外周动脉疾病模型中,长期饮酒会加剧缺血相关的骨骼肌线粒体功能障碍
目的外周动脉疾病(PAD)会导致与缺血性线粒体病相关的肌肉损伤,加重患者的残疾和死亡率。虽然酒精和高脂肪饮食会增加 PAD 的易感性和严重性,但它们对 PAD 肌病的影响尚不清楚。我们利用小鼠 PAD 模型,研究了长期饮酒和摄入脂肪对肌肉内氧化应激以及线粒体含量、功能和质量控制的综合影响。我们还探讨了肌肉内醛脱氢酶 2 (ALDH2) 含量、氧化应激和线粒体病变之间的潜在关系。方法24 只雄性、24 只雌性、8 个月大的 C57BL/6 J 小鼠接受高脂蔗糖 (HFS) 或低脂蔗糖 (LFS) 饮食 16 周,然后在饮用水中添加 20% 的乙醇 (EtOH) 或继续饮水 12 周(n = 12 只小鼠/4 组)。结扎左股动脉以诱导后肢缺血(HLI),结扎后 4 周的小鼠被安乐死。摄入 HFS + EtOH 的小鼠肌肉内 ALDH2 也减少了,尤其是缺血肢体,但摄入 LFS + EtOH 的小鼠肌肉内 ALDH2 增加了。此外,ALDH2的降低与氧化应激和线粒体功能障碍的标志物密切相关。结论ALDH2可能是优化PAD患者(尤其是习惯性摄入高脂肪和高酒精饮食的患者)肌肉内线粒体功能的一个很有前景的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


