The Al2O3-SiO2-“V2O3” phase diagram at 1873 K
IF 1.9
3区 材料科学
Q4 CHEMISTRY, PHYSICAL
Calphad-computer Coupling of Phase Diagrams and Thermochemistry
Pub Date : 2024-11-19
DOI:10.1016/j.calphad.2024.102771
引用次数: 0
Abstract
The pseudo-ternary Al2O3-SiO2-“V2O3” phase diagram was studied at 1873 K and oxygen partial pressures of 3.4 × 10−11, 3.3 × 10−10 and 3.4 × 10−9 atm. The samples were kept in a CO-CO2 mixture to control the oxygen potential and quenched in oil after equilibration. The phase compositions of the quenched samples were analyzed using wavelength-dispersive spectroscopy. The analyses were used to construct the phase diagrams. A change in oxygen potential did not profoundly affect the Al2O3-corundum + V2O3-corundum + mullite and V2O3-corundum + liquid + cristobalite equilibria, in contrast to the V2O3-corundum + mullite + liquid equilibrium where an effect was evident. For the latter equilibrium, decreased oxygen potential resulted in higher contents of Al2O3 and slightly higher contents of V2O3 in the liquid phase, as well as slightly higher contents of Al2O3 in V2O3-corundum and mullite.
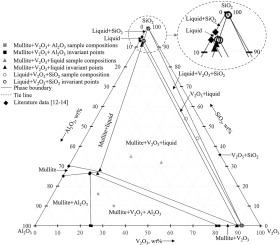
1873 K 时的 Al2O3-SiO2-"V2O3" 相图
在 1873 K 和氧分压分别为 3.4 × 10-11、3.3 × 10-10 和 3.4 × 10-9 atm 的条件下,研究了 Al2O3-SiO2-"V2O3 "伪三元相图。样品保存在 CO-CO2 混合物中以控制氧势,平衡后在油中淬火。使用波长色散光谱法分析了淬火样品的相组成。分析结果用于构建相图。氧势的变化对 Al2O3-刚玉 + V2O3-刚玉 + 莫来石和 V2O3-刚玉 + 液体 + 克里斯托巴利特的平衡影响不大,相反,对 V2O3-刚玉 + 莫来石 + 液体的平衡影响明显。在后一种平衡中,氧势降低导致液相中 Al2O3 含量增加,V2O3 含量略高,V2O3-刚玉和莫来石中 Al2O3 含量略高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.00
自引率
16.70%
发文量
94
审稿时长
2.5 months
期刊介绍:
The design of industrial processes requires reliable thermodynamic data. CALPHAD (Computer Coupling of Phase Diagrams and Thermochemistry) aims to promote computational thermodynamics through development of models to represent thermodynamic properties for various phases which permit prediction of properties of multicomponent systems from those of binary and ternary subsystems, critical assessment of data and their incorporation into self-consistent databases, development of software to optimize and derive thermodynamic parameters and the development and use of databanks for calculations to improve understanding of various industrial and technological processes. This work is disseminated through the CALPHAD journal and its annual conference.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


