2,3-diferrocenyl-(1-triphenylphosphoranylidene)ketene: Synthesis and interactions with O, C, N, S, Se nucleophiles, characterization and X-ray diffraction
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
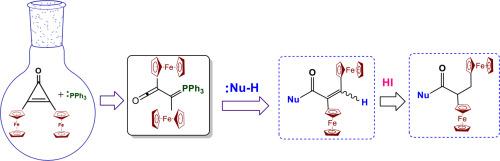
The opening of the ring of 2,3-diferrocenylcyclopropenone 1 with triphenylphosphine was studied to obtain 2,3-diferrocenyl-(3-triphenylphosphoranylidene) ketene 3. This was subsequently electrophile activated to react with different nucleophiles (O, C, N, S, Se) to obtain α,β-unsaturated carbonyl compounds with a preferable selectivity (E), which were stable under environmental conditions. The reduction reaction of the double bond of the α,β-unsaturated carbonyl compound was studied in the presence of hydrogen iodide. The structures of the synthesized compounds were established on the basis of data obtained from 1H and 13C NMR spectroscopy and further confirmed by X-ray diffraction analysis.
2,3-二茂苄基-(1-三苯基膦亚基)乙烯:合成、与 O、C、N、S、Se 亲核物的相互作用、表征和 X 射线衍射
通过研究 2,3-二茂铁基环丙烯酮 1 与三苯基膦的开环反应,得到 2,3-二茂铁基-(3-三苯基膦亚基) 烯酮 3。随后通过亲电活化,与不同的亲核物(O、C、N、S、Se)发生反应,得到α、β-不饱和羰基化合物,其选择性(E)更佳,且在环境条件下稳定。在碘化氢存在下,研究了 α、β-不饱和羰基化合物双键的还原反应。根据 1H 和 13C NMR 光谱数据确定了合成化合物的结构,并通过 X 射线衍射分析进一步证实了这些结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


