Modeling immune checkpoint inhibitor associated myocarditis in vitro and its therapeutic implications
Journal of molecular and cellular cardiology plus
Pub Date : 2024-11-20
DOI:10.1016/j.jmccpl.2024.100122
引用次数: 0
Abstract
Immune checkpoint inhibitor-associated myocarditis is the most lethal side effect of immune checkpoint blockade. Myocarditis leads to persistently increased mortality and lacks effective treatments. The development of patient-relevant disease models may enable disease prediction, increased understanding of disease pathophysiology, and the development of effective treatment strategies. Here, we report a new method to model immune checkpoint inhibitor-associated myocarditis in vitro via a co-culture of activated primary human immune cells, human induced pluripotent stem cell-derived cardiomyocytes, and FDA-approved immune checkpoint inhibitors to recapitulate myocarditis in vitro. Significant cardiomyocyte necrosis, arrhythmia development, and sarcomere destruction occur, replicating clinical findings from myocarditis. This tissue culture myocarditis phenotype may rely on an induced pluripotent stem cell-derived cardiomyocyte antigen-specific CD8+ T cell response. The administration of dexamethasone rescued cardiomyocyte viability, morphology, and electrophysiology and suppressed inflammatory cytokine production. In conclusion, we detail how this platform can effectively model and provide critical information about the morphological and electrophysiological changes induced by immune checkpoint inhibitor-associated myocarditis. We have also validated the ability of this platform to screen potential medications to treat immune checkpoint inhibitor-associated myocarditis. This work establishes a robust, scalable model for identifying new therapies and risk factors, which is valuable in delineating the nature of interactions between the immune system and the heart during myocarditis.
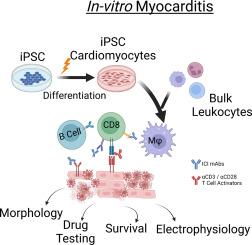
免疫检查点抑制剂相关心肌炎的体外建模及其治疗意义
免疫检查点抑制剂相关心肌炎是免疫检查点阻断疗法最致命的副作用。心肌炎导致死亡率持续上升,但缺乏有效的治疗方法。建立与患者相关的疾病模型可以预测疾病、加深对疾病病理生理学的了解并制定有效的治疗策略。在这里,我们报告了一种在体外建立免疫检查点抑制剂相关心肌炎模型的新方法,该方法通过将活化的原代人类免疫细胞、人类诱导多能干细胞衍生的心肌细胞和美国食品药品管理局批准的免疫检查点抑制剂进行联合培养来在体外再现心肌炎。心肌细胞出现明显坏死、心律失常和肌节破坏,再现了心肌炎的临床表现。这种组织培养心肌炎表型可能依赖于诱导多能干细胞衍生的心肌细胞抗原特异性 CD8+ T 细胞反应。给予地塞米松能挽救心肌细胞的活力、形态和电生理学,并抑制炎性细胞因子的产生。总之,我们详细介绍了该平台如何有效地模拟免疫检查点抑制剂相关心肌炎诱导的形态学和电生理学变化并提供相关重要信息。我们还验证了该平台筛选治疗免疫检查点抑制剂相关心肌炎潜在药物的能力。这项工作为确定新疗法和风险因素建立了一个稳健、可扩展的模型,这对于阐明心肌炎期间免疫系统与心脏之间相互作用的性质非常有价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of molecular and cellular cardiology plus
Cardiology and Cardiovascular Medicine
自引率
0.00%
发文量
0
审稿时长
31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


