Atomic mechanisms of oxidative behavior of ferrochromium alloys by water-oxygen environment
IF 3.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
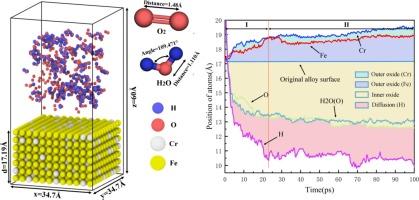
The formation of chromium-rich oxide layers on the surface of Fe-Cr alloys significantly impacts their performance at elevated temperatures. Understanding the formation process of these oxide layers at the atomic scale is crucial for further elucidating oxidation behavior, though it presents substantial challenges. In this study, ReaxFF molecular dynamics simulations were used to investigate the oxidation behavior of Fe-Cr alloys in high temperature water vapor and oxygen environments. The results demonstrate that chromium atoms are the primary contributors to the oxidation process, with Cr atoms diffusing to the surface more readily than iron atoms, leading to uneven stress distribution and creating high-stress regions near the Cr atoms. Additionally, hydrogen atoms generated from the breakdown of water molecules infiltrate the alloy matrix, promoting the creation, movement, and clustering of cation and anion vacancies, thereby enhancing oxidation reactions in high-temperature, humid conditions. The study further analyzes electron transfer during single-atom chemical reactions and explores the linear relationship between varying Cr content (10 %–30 %) and oxidation behavior under identical environmental conditions. These findings provide an important theoretical basis for optimizing the performance of Fe-Cr alloys for applications in high temperature environments.
水氧环境下铬铁合金氧化行为的原子机制
铁铬合金表面富铬氧化层的形成极大地影响了其在高温下的性能。在原子尺度上了解这些氧化层的形成过程对于进一步阐明氧化行为至关重要,但这也是一项巨大的挑战。本研究采用 ReaxFF 分子动力学模拟来研究铁铬合金在高温水蒸气和氧气环境中的氧化行为。结果表明,铬原子是氧化过程的主要成分,铬原子比铁原子更容易扩散到表面,从而导致应力分布不均匀,并在铬原子附近形成高应力区。此外,水分子分解产生的氢原子渗入合金基体,促进阳离子和阴离子空位的产生、移动和聚集,从而增强了高温潮湿条件下的氧化反应。研究进一步分析了单原子化学反应过程中的电子转移,并探讨了在相同环境条件下,不同铬含量(10%-30%)与氧化行为之间的线性关系。这些发现为优化铁铬合金在高温环境中的应用性能提供了重要的理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational Materials Science
工程技术-材料科学:综合
CiteScore
6.50
自引率
6.10%
发文量
665
审稿时长
26 days
期刊介绍:
The goal of Computational Materials Science is to report on results that provide new or unique insights into, or significantly expand our understanding of, the properties of materials or phenomena associated with their design, synthesis, processing, characterization, and utilization. To be relevant to the journal, the results should be applied or applicable to specific material systems that are discussed within the submission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


