Exploring the effect of temperature and peptide chain number on the stability of Aβ42 mutants through multidimensional analysis
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
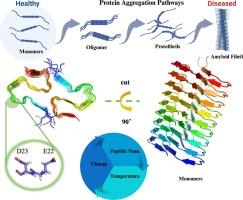
Amyloid beta 42 (Aβ42) plays a key role in the onset of Alzheimer’s Disease (AD), the seventh leading cause of death worldwide, and remains a focal point of related research. This study investigates familial mutations of Aβ42, including E22G (Arctic), E22K (Italian), E22Q (Dutch), and D23N (Iowa). These mutations, which involve changes in their single or double charges, markedly impact the structural stability and aggregation behavior of Aβ42. Using an enhanced precise polarized protein-specific charge method, this research delves deeply into the specific impacts of these mutations on the stability of oligomeric structures under different conditions, as well as the comprehensive effects of external factors such as temperature and internal factors including the number of peptide chains in complex on the structural stability and aggregation capability of Aβ42. This study shows that high temperatures generally compromise the stability and aggregation behavior of Aβ42, suggesting that moderately lower temperatures may better promote its stability. The principal component analysis supports this conclusion. At 400 and 450 K, despite a certain stability maintained by the polypeptide chains of Aβ42, high temperatures are unlikely to induce substantial conformational reorganization or folding of the protein. Binding free energy calculations reveal that as the number of peptide chains increases, complexes of 7- or 8-chain Aβ42 tend to stabilize, with minimal change in binding free energy as the number of peptide chains increases. Further analysis shows that at 250 K, the 9-chain E22Q, and at 350 K, 8-chain E22G, and the 7-chain D23N systems exhibit the most compact structures, suggesting that these systems are more prone to aggregation. Hydrophobicity and charge distribution analyses of the complexes also indicate that, compared to the wild type, the mutant systems demonstrate usually shows higher binding stability and aggregation potential. This study not only deepens our understanding of how temperature and the number of peptide chains affect familial mutations of Aβ42 but also emphasizes the importance of considering these microfactors in the research and treatment of AD.
通过多维分析探讨温度和肽链数对 Aβ42 突变体稳定性的影响
淀粉样蛋白β42(Aβ42)在阿尔茨海默病(AD)的发病中起着关键作用,是全球第七大死因,目前仍是相关研究的焦点。本研究调查了 Aβ42 的家族突变,包括 E22G(北极)、E22K(意大利)、E22Q(荷兰)和 D23N(爱荷华)。这些突变涉及单电荷或双电荷的变化,对 Aβ42 的结构稳定性和聚集行为有显著影响。本研究利用一种增强的精确极化蛋白质特异电荷方法,深入研究了这些突变在不同条件下对低聚物结构稳定性的具体影响,以及温度等外部因素和复合物中肽链数量等内部因素对 Aβ42 结构稳定性和聚集能力的综合影响。本研究表明,高温通常会影响 Aβ42 的稳定性和聚集行为,这表明适度降低温度可以更好地促进其稳定性。主成分分析支持这一结论。在 400 和 450 K 的温度下,尽管 Aβ42 的多肽链保持了一定的稳定性,但高温不太可能引起蛋白质的实质性构象重组或折叠。结合自由能计算显示,随着肽链数量的增加,7 链或 8 链 Aβ42 的复合物趋于稳定,随着肽链数量的增加,结合自由能的变化极小。进一步的分析表明,在 250 K 时,9 链 E22Q、350 K 时,8 链 E22G 和 7 链 D23N 系统的结构最为紧凑,表明这些系统更容易发生聚集。对复合物的疏水性和电荷分布分析也表明,与野生型相比,突变体系统通常表现出更高的结合稳定性和聚集潜力。这项研究不仅加深了我们对温度和肽链数量如何影响 Aβ42 家族突变的理解,还强调了在研究和治疗注意力缺失症时考虑这些微观因素的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


