Radical pathways for 2,4-chromandione synthesis via photoexcitation of 4-hydroxycoumarins
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
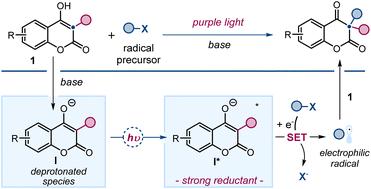
4-Hydroxycoumarins are well-known for their ground-state nucleophilic behavior, which has been widely exploited for their functionalization. Herein, we reveal a previously unexplored photochemical reactivity: upon deprotonation and excitation with purple light, 3-substituted 4-hydroxycoumarins reach an excited state and act as single-electron transfer (SET) reductants, generating radicals from stable substrates. This newfound reactivity enables the direct synthesis of 3,3-disubstituted 2,4-chromandiones via a radical dearomatization process. By enabling the incorporation of alkyl and perfluoroalkyl fragments, this protocol offers a straightforward and mild route to access synthetically valuable chromanone scaffolds featuring a quaternary stereocenter. Comprehensive photophysical studies confirmed that deprotonated 4-hydroxycoumarins are potent SET reductants in their excited state, making them suitable for initiating radical-based transformations.
通过光激发 4-羟基香豆素合成 2,4-香豆酮的辐射途径
4- 羟基香豆素以其基态亲核行为而闻名,这种行为已被广泛用于其功能化。在这里,我们揭示了一种以前从未探索过的光化学反应活性:在去质子化和紫光激发下,3-取代的 4-羟基香豆素会进入激发态,并作为单电子转移(SET)还原剂,从稳定的底物中生成自由基。这种新发现的反应活性使我们能够通过自由基脱芳烃过程直接合成 3,3-二取代的 2,4-色满二酮。通过加入烷基和全氟烷基片段,该方法提供了一种直接而温和的途径,以获得具有四元立体中心的、具有合成价值的铬烷酮支架。全面的光物理研究证实,去质子化的 4- 羟基香豆素在激发态下是强效的 SET 还原剂,因此适合用于启动基于自由基的转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


