A Complex Oxide Containing Inherent Peroxide Ions for Catalyzing Oxygen Evolution Reactions in Acid
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
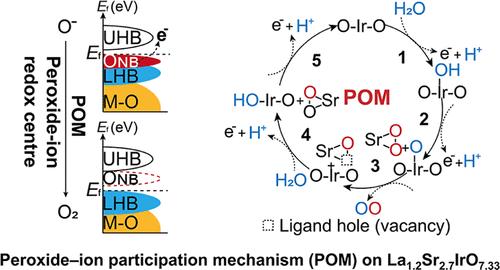
Proton exchange membrane water electrolyzers powered by sustainable energy represent a cutting-edge technology for renewable hydrogen generation, while slow anodic oxygen evolution reaction (OER) kinetics still remains a formidable obstacle that necessitates basic comprehension for facilitating electrocatalysts’ design. Here, we report a low-iridium complex oxide La1.2Sr2.7IrO7.33 with a unique hexagonal structure consisting of isolated Ir(V)O6 octahedra and true peroxide O22– groups as a highly active and stable OER electrocatalyst under acidic conditions. Remarkably, La1.2Sr2.7IrO7.33, containing 59 wt % less iridium relative to the benchmark IrO2, shows about an order of magnitude higher mass activity, 6-folds higher intrinsic activity than the latter, and also surpasses the state-of-the-art Ir-based oxides ever reported. Combined electrochemical, spectroscopic, and density functional theory investigations reveal that La1.2Sr2.7IrO7.33 follows the peroxide-ion participation mechanism under the OER condition, where the inherent peroxide ions with accessible nonbonded oxygen states are responsible for the high OER activity. This discovery offers an innovative strategy for designing advanced catalysts for various catalytic applications.
一种含有固有过氧离子的复合氧化物,用于催化酸中的氧进化反应
以可持续能源为动力的质子交换膜水电解槽是可再生制氢的前沿技术,而缓慢的阳极氧进化反应(OER)动力学仍然是一个巨大的障碍,需要对其进行基本了解,以促进电催化剂的设计。在此,我们报告了一种低铱复合氧化物 La1.2Sr2.7IrO7.33,它具有独特的六边形结构,由孤立的 Ir(V)O6 八面体和真正的过氧化物 O22- 基团组成,是一种在酸性条件下具有高活性和稳定性的 OER 电催化剂。值得注意的是,与基准氧化铱相比,La1.2Sr2.7IrO7.33 的铱含量减少了 59 wt %,但其质量活性却比后者高出约一个数量级,本征活性比后者高出 6 倍,而且还超过了迄今为止所报道的最先进的铱基氧化物。电化学、光谱和密度泛函理论的综合研究表明,La1.2Sr2.7IrO7.33 在 OER 条件下遵循过氧化物-离子参与机制,其中具有可访问非键氧态的固有过氧化物离子是产生高 OER 活性的原因。这一发现为设计用于各种催化应用的先进催化剂提供了一种创新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


