Precisely Controlling the Activation of an Iron-Locked Drug Generator in the Liver Sinusoid to Enhance Barrier Penetration and Reduction of Liver Fibrosis
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
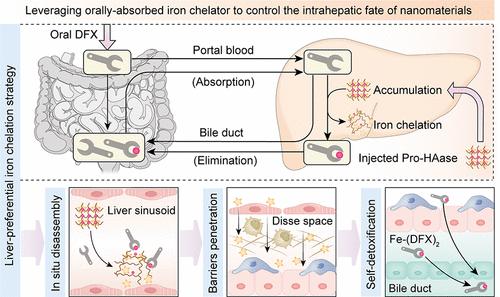
Complex physical barriers and the nanomaterial’s clearance mechanism in the liver greatly hinder the feasibility of using a conventional liver-targeting nanoplatform to deliver antifibrotic drugs to pathological sites for the treatment of liver fibrosis. Here, a novel drug delivery strategy was designed to overcome drug penetration barriers in a fibrotic liver and cooperated with oral nattokinase (NKase)-mediated antifibrosis therapy as a proof of concept, which relies on the coadministration of a nanosized iron-locked drug generator (named Pro-HAase) and orally absorbed iron chelator deferasirox (DFX). Such a strategy starts from the rapid accumulation of intravenously injected Pro-HAase in the microcapillaries of the fibrotic liver followed by disrupting the polyphenol-iron coordination inside Pro-HAase by DFX, liberating antifibrotic components, including procyanidine (PA) and hyaluronidase (HAase). Attractively, absorption of DFX requires the sequential processes of traversing the intestinal mucosa and targeting the liver, which enable DFX to preferentially disassemble Pro-HAase accumulated in the liver sinusoid rather than in systemic circulation or other organs, thus avoiding the off-target activation of Pro-HAase and depletion of the normal iron pool. The in situ disassembly process decreases the sequestration of Pro-HAase by cells of the mononuclear phagocyte system and promotes gradient-driven permeation of therapeutic components to surrounding liver tissues within 2 h, accompanied by biliary excretion of the inactive iron-DFX complex. As a result, the cooperation of Pro-HAase and DFX not only allows NKase-mediated therapy to completely reverse liver fibrosis but also suppresses the chronic hepatotoxicity of residual liver iron after multiple doses of Pro-HAase. The high spatiotemporal precision, unique barrier-penetration mechanism, and self-detoxification ability of this strategy will inspire the rational design of analogous iron-locked nanosystems to improve the therapeutic outcomes of liver fibrosis or other liver diseases.
精确控制肝窦内铁锁药物发生器的活化以增强屏障穿透力和减轻肝纤维化
复杂的物理障碍和纳米材料在肝脏中的清除机制极大地阻碍了利用传统的肝脏靶向纳米平台将抗肝纤维化药物输送到病理部位以治疗肝纤维化的可行性。在这里,我们设计了一种新型给药策略,以克服药物在纤维化肝脏中的渗透障碍,并配合口服纳豆激酶(NKase)介导的抗纤维化治疗作为概念验证,该策略依赖于纳米尺寸的铁锁定药物发生器(命名为Pro-HAase)和口服铁螯合剂地拉罗司(DFX)的联合给药。这种策略的起点是静脉注射的 Pro-HAase 在纤维化肝脏的微毛细血管中快速聚集,然后 DFX 破坏 Pro-HAase 内的多酚铁配位,释放抗纤维化成分,包括丙氰苷(PA)和透明质酸酶(HAase)。吸引人的是,DFX 的吸收需要经过穿越肠粘膜和靶向肝脏的连续过程,这使得 DFX 能够优先分解积聚在肝脏窦状结构中的 Pro-HAase,而不是全身循环或其他器官中的 Pro-HAase,从而避免了 Pro-HAase 的脱靶激活和正常铁池的耗竭。原位分解过程减少了单核吞噬细胞系统细胞对 Pro-HAase 的封存,促进了治疗成分在 2 小时内梯度渗透到肝脏周围组织,并伴随着无活性铁-DFX 复合物的胆汁排泄。因此,Pro-HAase 和 DFX 的合作不仅能使 NKase 介导的疗法完全逆转肝纤维化,还能抑制多剂量 Pro-HAase 后残留肝铁的慢性肝毒性。该策略的高时空精确性、独特的屏障渗透机制和自我解毒能力将启发人们合理设计类似的锁铁纳米系统,以改善肝纤维化或其他肝病的治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
公司名称
产品信息
索莱宝
Masson trichrome staining kit
索莱宝
collagenase IV
索莱宝
collagenase I

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


