Hydration-Shell Solvation and Screening Govern Alkali Cation Concentrations at Electrochemical Interfaces
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Knowledge of the concentration of alkali cations in an electrochemical double layer is essential for interpreting and leveraging cation effects in electrocatalysis. We systematically study the concentration profiles of four alkali cations (Li+, Na+, K+, and Cs+) at a Ag(111)-aqueous interface. Using classical molecular dynamics, the potential of mean force (PMF) of cations approaching a metal surface was computed and decomposed into contributions from the solvent and the metal surface. We find that hydration shell deformations contribute importantly to the free energy of cations near the electrode. Cations with larger ionic radii and looser hydration shells experience less solvation loss and less short-range Coulombic screening, which enable them to adsorb more strongly to a negatively charged surface (Cs+ > K+ > Na+ > Li+). We compute the non-Faradaic electrosorption valency and the interfacial capacitance and show that these experimentally relevant quantities encode the relative concentration of the adsorbed alkali cations of different sizes, but not the spatial positions of cations in the double layer.
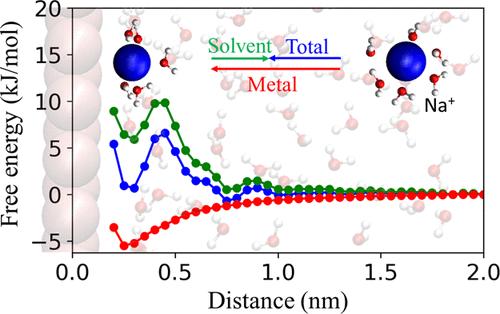
水合壳溶解和筛选治理电化学界面的碱阳离子浓度
了解电化学双层中碱阳离子的浓度对于解释和利用电催化中的阳离子效应至关重要。我们系统研究了 Ag(111)- 水界面上四种碱阳离子(Li+、Na+、K+ 和 Cs+)的浓度分布。利用经典分子动力学计算了阳离子接近金属表面时的平均力势(PMF),并将其分解为来自溶剂和金属表面的贡献。我们发现,水合壳变形对靠近电极的阳离子的自由能有重要影响。离子半径较大、水合壳较松散的阳离子经历的溶解损失和短程库仑筛选较少,这使它们能够更强地吸附到带负电荷的表面(Cs+ > K+ > Na+ > Li+)。我们计算了非法拉第电吸附价和界面电容,并表明这些与实验相关的量编码了吸附的不同大小碱阳离子的相对浓度,而不是阳离子在双电层中的空间位置。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


