Depression decreases immunity and PD-L1 inhibitor efficacy via the hypothalamic–pituitary–adrenal (HPA) axis in triple-negative breast cancer
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-11-22
DOI:10.1016/j.bbadis.2024.167581
引用次数: 0
Abstract
Background
Depression weakens antitumor immunity, yet the underlying mechanisms linking depression and tumor growth remain unclear. This study examines the influence of depression on the hypothalamic–pituitary–adrenal (HPA) axis, immunological function, and effectiveness of immunotherapy in triple-negative breast cancer (TNBC) patients.
Methods
A mouse model of comorbid TNBC and depression was established via chronic restraint stress (CRS) and 4T1 tumor transplantation. A programmed cell death ligand 1 (PD-L1) inhibitor was used to manage mice with TNBC, and the ability of metyrapone to reverse the immune system changes induced by HPA axis activation in depression was evaluated. Mouse peripheral blood was used to measure HPA axis activity, immune cell numbers and cytokine levels.
Results
Depression activates the HPA axis, leading to increased levels of glucocorticoids. Depression led to an increase in the B-cell number and a reduction in the CD4+ T-cell and CD8+ T-cell numbers, without a statistically significant difference in the regulatory T (Treg) cell number. Furthermore, depression increased the levels of the cytokines interferon-gamma (IFN-γ), interleukin (IL)-1β, IL-4, IL-6, IL-8, and tumor necrosis factor (TNF)-α while decreasing the levels of IL-2 and IL-10. Similar results were observed in the context of PD-L1 inhibitor therapy. The depressed mice presented an increased tumor burden and a poor response to the PD-L1 inhibitor. The application of metyrapone during PD-L1 inhibitor treatment resulted in partial restoration of these depression-related alterations.
Conclusions
Depression reduces the effectiveness of PD-L1 inhibitors by altering the number of immune cells and the levels of cytokines through activation of the HPA axis.
Translational relevance
Depression is common in breast cancer patients and is associated with reduced antitumor immunity. There is limited knowledge regarding the specific mechanisms through which depression impairs antitumor immunity. Immunotherapy, which promotes the restoration of antitumor immunity, represents a promising treatment strategy for TNBC patients. However, the efficacy of immunotherapy can be compromised by depressive symptoms and the administration of glucocorticoids during treatment. It is still uncertain whether increasing glucocorticoid levels can reduce the efficacy of immunotherapy in patients with depression. The potential benefits of combining immunotherapy with glucocorticoid inhibitors compared with immunotherapy alone need to be evaluated for TNBC patients with concurrent depressive symptoms. Therefore, further clarification of the specific mechanisms by which depression impairs antitumor immunity is needed to inform future optimization of immunotherapy strategies.
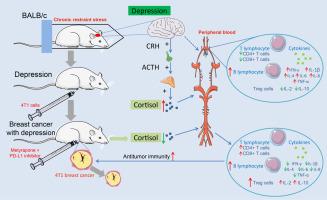
抑郁症会通过下丘脑-垂体-肾上腺(HPA)轴降低三阴性乳腺癌患者的免疫力和PD-L1抑制剂的疗效。
背景:抑郁症会削弱抗肿瘤免疫力,但抑郁症与肿瘤生长之间的内在机制仍不清楚。本研究探讨了抑郁症对三阴性乳腺癌(TNBC)患者下丘脑-垂体-肾上腺(HPA)轴、免疫功能和免疫治疗效果的影响:方法:通过慢性束缚应激(CRS)和4T1肿瘤移植建立了TNBC和抑郁症的小鼠模型。方法:通过慢性束缚应激(CRS)和4T1肿瘤移植建立了TNBC和抑郁症小鼠模型,使用程序性细胞死亡配体1(PD-L1)抑制剂管理TNBC小鼠,并评估了metyrapone逆转抑郁症HPA轴激活诱导的免疫系统变化的能力。小鼠外周血用于测量HPA轴活性、免疫细胞数量和细胞因子水平:结果:抑郁症激活了 HPA 轴,导致糖皮质激素水平升高。抑郁症导致 B 细胞数量增加,CD4+ T 细胞和 CD8+ T 细胞数量减少,但调节性 T(Treg)细胞数量的差异无统计学意义。此外,抑郁症还会增加细胞因子γ干扰素(IFN-γ)、白细胞介素(IL)-1β、IL-4、IL-6、IL-8和肿瘤坏死因子(TNF)-α的水平,同时降低IL-2和IL-10的水平。在PD-L1抑制剂疗法中也观察到了类似的结果。抑郁小鼠的肿瘤负荷增加,对PD-L1抑制剂的反应不佳。在PD-L1抑制剂治疗期间应用甲萘醌可部分恢复这些抑郁相关的改变:抑郁症通过激活HPA轴改变免疫细胞的数量和细胞因子的水平,从而降低PD-L1抑制剂的疗效:抑郁症在乳腺癌患者中很常见,与抗肿瘤免疫力下降有关。人们对抑郁症损害抗肿瘤免疫力的具体机制了解有限。免疫疗法可促进抗肿瘤免疫力的恢复,是治疗 TNBC 患者的一种前景广阔的治疗策略。然而,抑郁症状和治疗期间糖皮质激素的使用可能会影响免疫疗法的疗效。目前还不确定增加糖皮质激素水平是否会降低抑郁症患者的免疫疗法疗效。对于同时伴有抑郁症状的 TNBC 患者,需要评估将免疫疗法与糖皮质激素抑制剂联合使用与单独使用免疫疗法相比的潜在益处。因此,需要进一步明确抑郁症损害抗肿瘤免疫力的具体机制,为今后优化免疫疗法策略提供依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


