CtBP1 is essential for epigenetic silencing of μ-opioid receptor genes in the dorsal root ganglion in spinal nerve ligation-induced neuropathic pain
IF 6.9
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
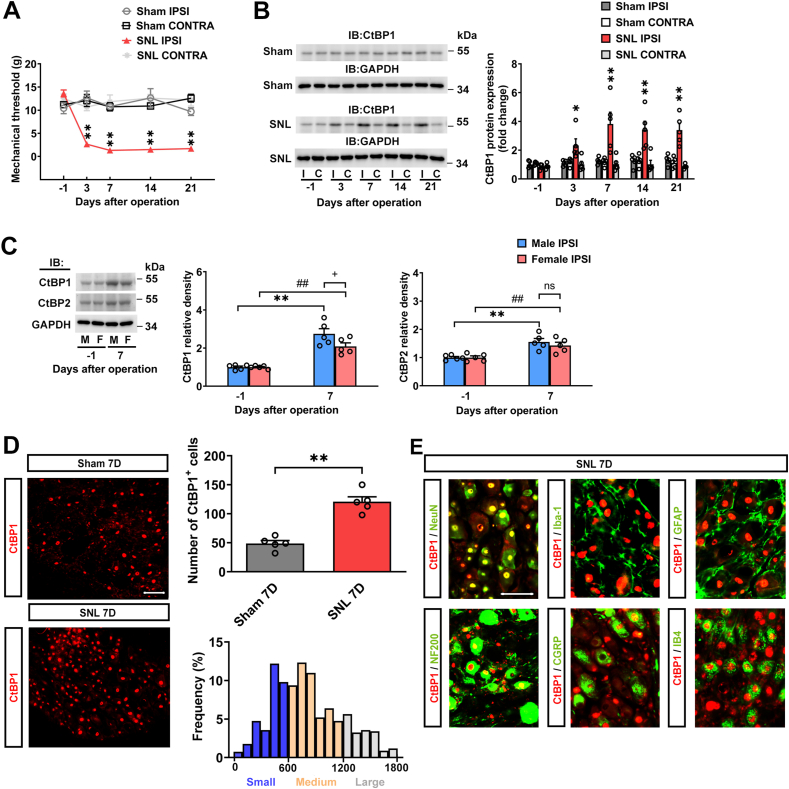
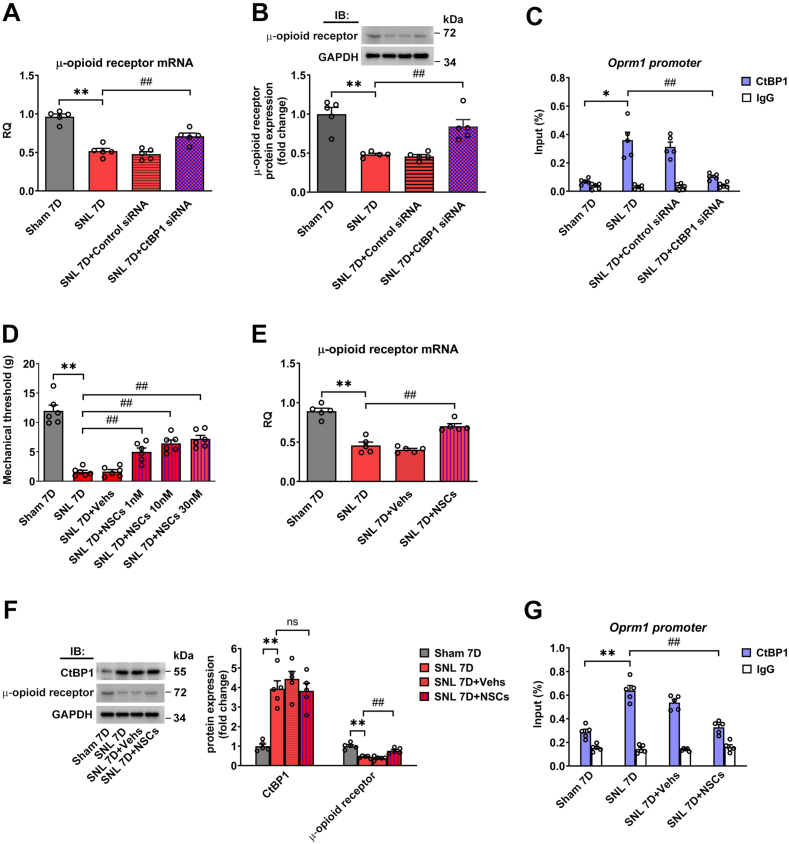
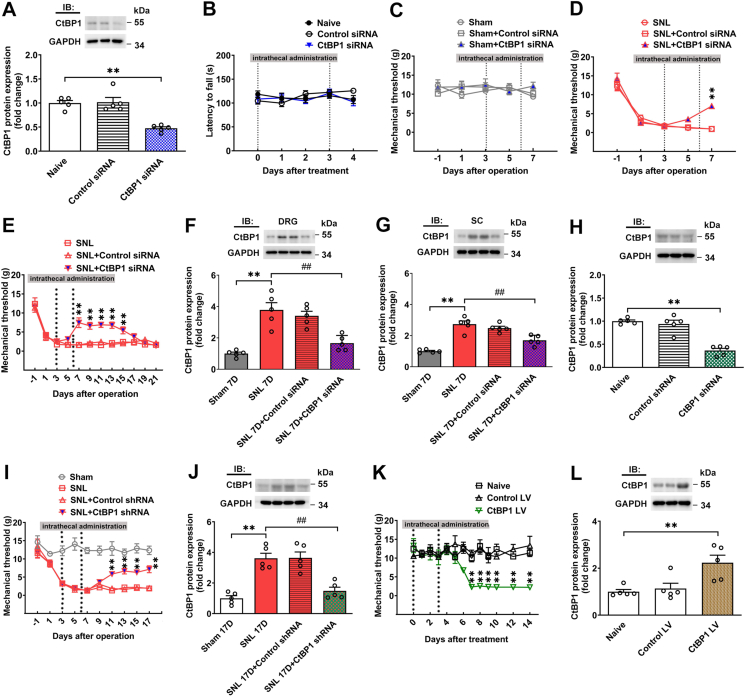
Neuropathic pain poses a significant public health challenge, greatly impacting patients' quality of life. Emerging evidence underscores the involvement of epigenetics in dorsal root ganglion (DRG) neurons relevant to pain modulation. C-terminal binding protein 1 (CtBP1) has emerged as a crucial epigenetic transcriptional coregulator. However, the underlying molecular mechanisms of CtBP1-mediated epigenetic regulation in DRG neurons in neuropathic pain remain poorly elucidated. Here, we employed a Sprague‒Dawley rat model of spinal nerve ligation (SNL) to establish a neuropathic pain model. CtBP1 expression in the ipsilateral DRG gradually increased over a three-week period post-SNL. Immunohistochemistry revealed a significant elevation in CtBP1 levels specifically in NeuN-positive neuronal cells in the ipsilateral DRG following SNL. Further characterization demonstrated CtBP1 expression across various subtypes of DRG neurons in SNL rats. Silencing CtBP1 expression with siRNA reversed tactile allodynia in SNL rats and restored both CtBP1 and μ-opioid receptor expression in the DRG in SNL rats. Moreover, Foxp1 was identified to recruit CtBP1 for mediating μ-opioid receptor gene silencing in the DRG in SNL rats. Subsequent investigation unveiled that Foxp1 recruits CtBP1 and associates with HDAC2 to regulate H3K9Ac binding to μ-opioid receptor chromatin regions in the DRG in SNL rats, implicating epigenetic mechanisms in neuropathic pain. Targeting the Foxp1/CtBP1/HDAC2/μ-opioid receptor signaling pathway in the DRG holds promise as a potential therapeutic strategy for managing neuropathic pain.
CtBP1对脊神经结扎诱导的神经病理性疼痛中背根神经节中μ-阿片受体基因的表观遗传沉默至关重要。
神经病理性疼痛是一项重大的公共卫生挑战,极大地影响了患者的生活质量。新出现的证据强调,背根神经节(DRG)神经元中的表观遗传学参与了疼痛调节。C-terminal binding protein 1(CtBP1)已成为一种重要的表观遗传转录核心调节因子。然而,CtBP1 介导的神经病理性疼痛 DRG 神经元表观遗传调控的潜在分子机制仍未得到充分阐明。在此,我们采用 Sprague-Dawley 大鼠脊神经结扎(SNL)模型建立了神经病理性疼痛模型。在SNL后的三周内,同侧DRG中的CtBP1表达量逐渐增加。免疫组化显示,SNL后同侧DRG中NeuN阳性神经元细胞的CtBP1水平显著升高。进一步的特征研究表明,CtBP1 的表达遍及 SNL 大鼠 DRG 神经元的各种亚型。用 siRNA 沉默 CtBP1 的表达可逆转 SNL 大鼠的触觉过敏症,并恢复 SNL 大鼠 DRG 中 CtBP1 和 μ - 阿片受体的表达。此外,研究还发现 Foxp1 能招募 CtBP1 来介导 SNL 大鼠 DRG 中的μ-阿片受体基因沉默。随后的研究发现,Foxp1会招募CtBP1并与HDAC2结合,以调节H3K9Ac与SNL大鼠DRG中的μ-阿片受体染色质区域的结合,从而揭示了神经病理性疼痛的表观遗传机制。靶向DRG中的Foxp1/CtBP1/HDAC2/μ-阿片受体信号通路有望成为治疗神经病理性疼痛的一种潜在治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurotherapeutics
医学-神经科学
CiteScore
11.00
自引率
3.50%
发文量
154
审稿时长
6-12 weeks
期刊介绍:
Neurotherapeutics® is the journal of the American Society for Experimental Neurotherapeutics (ASENT). Each issue provides critical reviews of an important topic relating to the treatment of neurological disorders written by international authorities.
The Journal also publishes original research articles in translational neuroscience including descriptions of cutting edge therapies that cross disciplinary lines and represent important contributions to neurotherapeutics for medical practitioners and other researchers in the field.
Neurotherapeutics ® delivers a multidisciplinary perspective on the frontiers of translational neuroscience, provides perspectives on current research and practice, and covers social and ethical as well as scientific issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


