Multispectral analysis and molecular docking of a zinc (II) complex interaction with bovine serum albumin and studies on antibacterial properties, and catecholase mimicry of the complex
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
This paper presents the synthesis process of a ligand known as 2-(naphthalene-1-yl)-1H-phenanthro[9,10-d]imidazole (NIP) and its metal complex with zinc (II), denoted as FA-128. The structural validation of FA-128 is accomplished through single-crystal X-ray diffraction (XRD). To explore the biological implications, FA-128's interaction with BSA is investigated. This exploration involves fluorescence and UV–vis absorption spectrometry techniques. The outcomes reveal the formation of robust complexes, as FA-128 significantly quenches the inherent fluorescence of BSA. Various aspects are examined, including binding constants, the count of binding sites, thermodynamic parameters, and energy transfer mechanisms. Evident alterations in BSA conformation are detected using synchronous fluorescence and circular dichroism (CD) spectrum techniques. The study proceeds to molecular docking, elucidating binding sites in the FA-128-BSA interaction. Biochemical reactions between metal complexes and proteins often trigger diverse conformational changes in protein structures. This understanding provides crucial insights into the impacts, mechanisms, and systemic transportation of numerous drugs within the body. FA-128 demonstrated superior antibacterial activity against Staphylococcus aureus (ZOI: 10.50 ± 0.50 mm, MIC: 100 μg/mL) and Klebsiella pneumoniae (ZOI: 13.0 ± 0.25 mm, MIC: 50 μg/mL). In addition, FA-128 has been evaluated as a catalytic system in the oxidation of 3,5-di-tert-butylcatechol (3,5DTBC) in a methanol solvent. FA-128 displays good catecholase-like activity with a significant turnover number (kcat) of 7.56 × 102 h−1, a Michaelis-Menten constant (KM) of 8.14 × 10−4 M, and a maximum reaction rate (Vmax) of 2.45 × 10−5 M s−1 under aerobic conditions.
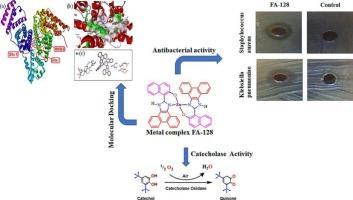
锌 (II) 复合物与牛血清白蛋白相互作用的多光谱分析和分子对接,以及对该复合物抗菌特性和儿茶酚酶模拟作用的研究。
本文介绍了一种名为 2-(萘-1-基)-1H-菲啰并[9,10-d]咪唑(NIP)的配体及其与锌(II)的金属络合物(代号为 FA-128)的合成过程。FA-128 的结构验证是通过单晶 X 射线衍射 (XRD) 完成的。为了探索其生物学意义,研究了 FA-128 与 BSA 的相互作用。这项研究涉及荧光和紫外-可见吸收光谱分析技术。研究结果表明,FA-128 能显著淬灭 BSA 的固有荧光,从而形成稳定的复合物。研究考察了各个方面,包括结合常数、结合位点数量、热力学参数和能量传递机制。利用同步荧光和圆二色性(CD)光谱技术检测了 BSA 构象的明显变化。研究还进行了分子对接,阐明了 FA-128 与 BSA 相互作用的结合位点。金属复合物与蛋白质之间的生化反应通常会引发蛋白质结构的多种构象变化。这种认识为了解多种药物在体内的影响、机制和系统运输提供了至关重要的见解。FA-128 对金黄色葡萄球菌(ZOI:10.50 ± 0.50 mm,MIC:100 μg/mL)和肺炎克雷伯菌(ZOI:13.0 ± 0.25 mm,MIC:50 μg/mL)具有卓越的抗菌活性。此外,还评估了 FA-128 作为催化系统在甲醇溶剂中氧化 3,5-二叔丁基邻苯二酚(3,5DTBC)的效果。在有氧条件下,FA-128 表现出良好的儿茶酚酶样活性,其显著周转数(kcat)为 7.56 × 102 h-1,迈克尔斯-门顿常数(KM)为 8.14 × 10-4 M,最大反应速率(Vmax)为 2.45 × 10-5 M s-1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


