Exploring flavonoids as potent SLC46A3 inhibitors: Insights from the structural characteristics of flavonoid–SLC46A3 interactions
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
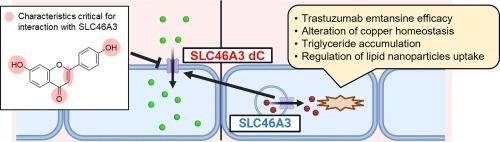
SLC46A3, a transporter for lysosomal steroid conjugates and bile acids, plays a pivotal role in the pharmacological effects of noncleavable antibody–drug conjugates using maytansine as a payload. SLC46A3 may exert negative effects on various phenomena, including copper homeostasis, mitochondrial function in the liver, and the uptake of lipid-based nanoparticles (NPs) in tumor cells. Consequently, inhibiting SLC46A3 may be a promising strategy for treating hepatic disease or enhancing lipid NP delivery to tumor cells, although the underlying mechanisms remain unknown. This study investigates flavonoids, the largest subgroup of polyphenols characterized by a simple C6-C3-C6 structure, as potential SLC46A3 inhibitors and provides insights into the structural requirements for flavonoid–SLC46A3 interactions. Screening revealed several flavonoids, including dihydrochalcones, flavonols, isoflavones, flavanones, and flavones, as effective inhibitors of 5-carboxyfluorescein (5-CF) uptake in MDCKII (Mardin-Darby canine kidney type II) cells stably expressing a mutant SLC46A3 localized to the plasma membrane. Notably, apigenin and luteolin exhibited marked 5-CF uptake inhibition, with IC50 values of 10.8 and 8.7 µM, respectively. Additionally, 4′,7-dihydroxyflavone significantly inhibited 5-CF uptake, exhibiting an IC50 value of 9.3 µM, whereas acacetin and genkwanin possessing methoxy group substitutions for the hydroxy group at the 4′- or 7-position of apigenin, respectively, did not affect the uptake. Luteolin’s inhibition mechanism was found to be of a mixed type involving increased Km and decreased Vmax. These findings emphasize the importance of hydroxy groups at 4′- and 7-positions in flavone–SLC46A3 interactions.
探索黄酮类化合物作为有效的 SLC46A3 抑制剂:从类黄酮-SLC46A3相互作用的结构特征中获得启示。
SLC46A3是溶酶体类固醇共轭物和胆汁酸的转运体,它在以maytansine为有效载荷的不可清除抗体药物共轭物的药理作用中起着关键作用。SLC46A3 可能会对多种现象产生负面影响,包括铜平衡、肝脏线粒体功能以及肿瘤细胞对脂质纳米颗粒(NPs)的吸收。因此,抑制 SLC46A3 可能是治疗肝病或增强脂质 NP 向肿瘤细胞递送的一种有前途的策略,尽管其潜在机制仍然未知。黄酮类化合物是多酚类化合物中最大的亚类,具有简单的 C6-C3-C6 结构,本研究将其作为潜在的 SLC46A3 抑制剂进行研究,并深入了解黄酮类化合物与 SLC46A3 之间相互作用的结构要求。筛选发现了几种黄酮类化合物,包括二氢查耳酮、黄酮醇、异黄酮、黄烷酮和黄酮,它们是在稳定表达定位于质膜的突变型 SLC46A3 的 MDCKII(马丁-达比犬肾 II 型)细胞中摄取 5-羧基荧光素(5-CF)的有效抑制剂。值得注意的是,芹菜素和木犀草素表现出明显的 5-CF 摄取抑制作用,IC50 值分别为 10.8 µM 和 8.7 µM。此外,4',7-二羟基黄酮也能显著抑制 5-CF 的摄取,其 IC50 值为 9.3 µM,而芹菜素和 Genkwanin(分别在芹菜素的 4'- 或 7 位羟基上具有甲氧基取代基团)则不影响 5-CF 的摄取。研究发现,木犀草素的抑制机制属于混合型,包括 Km 增加和 Vmax 降低。这些发现强调了4'-和7-位羟基在黄酮-SLC46A3相互作用中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


