Mechanistic differences between the Ru(ii) and Zn(ii)-catalyzed cross-coupling of cyclopropenes with diazo compounds: a DFT study†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Transition-metal-catalyzed cross-coupling of two different carbene precursors has been established to construct olefins. In this context, Ru(ii) and Zn(ii)-catalyzed cross-coupling of cyclopropenes with diazo compounds to synthesize 1,3-butadiene derivatives has been developed. Herein, a detailed computational study was performed to shed light on the mechanistic differences between the Ru(ii) and Zn(ii)-catalyzed cross-coupling of cyclopropenes with diazo compounds. For the Ru(ii)-catalyzed reaction, the formation of an Ru–carbene intermediate with diazo compounds is more feasible. Next, cyclopropenes could undergo a [2 + 2] cycloaddition with the Ru–carbene intermediate to form a four-membered ring intermediate, from which an olefin metathesis mechanistic pathway is suggested. Afterward, an unusual alkenyl 1,2-migration might occur to afford the desired cross-coupling product. Meanwhile, for the Zn(ii)-catalyzed reaction, the formation of Zn–carbenoid with cyclopropenes more readily occurs. Then, the nucleophilic C-attack of diazo compounds to the carbene moiety is suggested, leading to the desired product in a concerted manner. The origin of the different chemoselectivities in activating cyclopropenes/diazo compound carbene precursors for the Ru(ii) and Zn(ii) catalysis was discussed.
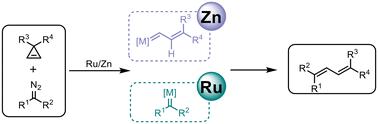
Ru(ii)和 Zn(ii)催化的环丙烯与重氮化合物交叉偶联的机理差异:一项 DFT 研究†。
过渡金属催化两种不同碳烯前体的交叉偶联已被证实可以生成烯烃。在此背景下,开发了 Ru(II) 和 Zn(II) 催化的环丙烯与重氮化合物的交叉偶联反应,以合成 1,3-丁二烯衍生物。在此,我们进行了详细的计算研究,以揭示 Ru(II) 和 Zn(II) 催化的环丙烯与重氮化合物交叉偶联的机理差异。在 Ru(II) 催化的反应中,Ru-烯中间体与重氮化合物的形成更为可行。接下来,环丙烯可能与 Ru-烯中间体发生[2 + 2]环加成反应,形成四元环中间体,并由此提出了烯烃元合成的机理途径。之后,可能会发生不寻常的烯基 1,2-迁移,从而得到所需的交叉耦合产物。同时,在 Zn(II)催化的反应中,Zn-羰基与环丙烯的形成更容易发生。然后,重氮化合物对碳烯分子的亲核 C 攻击被提出,从而以协同方式得到所需的产物。讨论了 Ru(II) 和 Zn(II) 催化活化环丙烯/重氮化合物碳烯前体时产生不同化学选择性的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


