Improved charge carrier mobility in a copper oxide heterostructure enhances the photocatalytic partial oxidation of benzyl alcohol to benzaldehyde†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Copper oxides have been studied over the past decade for their potential in heterogeneous photocatalysis. However, fundamental issues regarding charge carrier behavior remain largely unexplored. In this study, we investigate the charge carrier mobility of CuO, CuO/Cu2O, and Cu2O using time-resolved microwave conductivity in relation to their photocatalytic performance in the partial oxidation of benzyl alcohol to benzaldehyde under visible light at 455 nm. The photoconductivity-lifetime product shows a good correlation with the conversion of benzyl alcohol and the yield of benzaldehyde, with CuO/Cu2O exhibiting the highest performance. The favorable behavior of charge carriers in CuO/Cu2O for photocatalysis is attributed to the presence of CuO impurity, which enables more efficient separation of photoexcited electrons and holes via the S-scheme heterojunction. The charge carrier mobility and photocatalytic performance are strongly influenced by the optoelectronic properties rather than by physical properties. By introducing a small amount of CuO (5.3 mol% based on X-ray absorption near edge structure), the charge carrier mobility of Cu2O is improved, enhancing performance even when the specific surface area decreases. Based on the photoconductivity-lifetime product, we reveal how a heterostructured photocatalyst exhibits enhanced performance. This photophysical parameter is a promising indicator for evaluating and designing future photocatalysts.
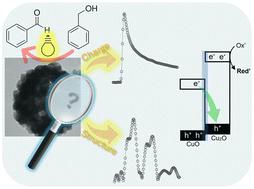
氧化铜异质结构中电荷载流子迁移率的提高增强了苯甲醇到苯甲醛的光催化部分氧化作用†。
过去十年来,人们一直在研究铜氧化物在异相光催化方面的潜力。然而,有关电荷载流子行为的基本问题在很大程度上仍未得到探讨。在本研究中,我们利用时间分辨微波电导率研究了 CuO、CuO/Cu2O 和 Cu2O 的电荷载流子迁移率与它们在 455 纳米可见光下将苯甲醇部分氧化为苯甲醛的光催化性能的关系。光电导寿命乘积与苯甲醇的转化率和苯甲醛的产率有很好的相关性,其中 CuO/Cu2O 表现出最高的性能。CuO/Cu2O 中电荷载流子在光催化中的良好表现归因于 CuO 杂质的存在,它通过 S 型异质结实现了光激发电子和空穴的更有效分离。电荷载流子迁移率和光催化性能主要受光电特性而非物理性质的影响。通过引入少量的 CuO(根据 X 射线吸收近边缘结构为 5.3 摩尔%),Cu2O 的电荷载流子迁移率得到了改善,即使比表面积减小,其性能也得到了提高。基于光导率-寿命乘积,我们揭示了异质结构光催化剂是如何提高性能的。这一光物理参数是评估和设计未来光催化剂的一个很有前途的指标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


