Mechanistic insights into the oxidative coupling of methane over a Li/MgO catalyst: an experimental and microkinetic modeling study†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
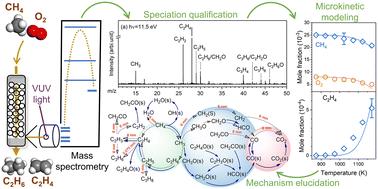
This study investigates the oxidative coupling of methane (OCM) using a Li/MgO catalyst in a packed bed reactor. Experiments were conducted at a pressure of 110 Torr over a temperature range of 873–1173 K. Stable products, including alcohols, aldehydes, ketones, and C3 hydrocarbons, were quantified using synchrotron vacuum ultraviolet photoionization mass spectrometry (SVUV-PIMS). Methyl and ethyl radicals were definitively identified, emphasizing their critical role in the formation of C2–C3 hydrocarbons. Based on these speciation results, a microkinetic model for the OCM reaction over Li/MgO was developed. The reaction network analysis revealed that the main pathway for COx generation involves the conversion of CH3 to CH3O(s)/CH3O through surface and gas-phase reactions. Gas-phase reactions also promote the deep oxidation of C2H4. Sensitivity analysis indicated that methane activation is primarily governed by surface reactions, while the coupling of CH3 to form C2H6 is mainly driven by gas-phase reactions. Both surface and gas-phase reactions contribute equally to the formation of C2H4. Additionally, the pressure dependence analysis demonstrated that the high-pressure limit of the CH3 coupling reaction restricts the increase in C2 yield as pressure rises. In conclusion, this study provides a comprehensive investigation into the OCM speciation pool, develops a microkinetic model aligned with experimental findings, and elucidates the reaction network driven by free radicals. These insights offer valuable guidance for optimization reaction conditions and catalyst performance.
锂/氧化镁催化剂上甲烷氧化偶联的机理研究:实验和微动力学模型研究†。
本研究调查了在填料床反应器中使用锂/氧化镁催化剂进行甲烷氧化偶联(OCM)的情况。使用同步辐射真空紫外光离子化质谱(SVUV-PIMS)对包括醇、醛、酮和 C3 碳氢化合物在内的稳定产物进行了定量。确定了甲基和乙基自由基,强调了它们在 C2-C3 碳氢化合物形成过程中的关键作用。根据这些标样结果,建立了锂/氧化镁上 OCM 反应的微动力学模型。反应网络分析显示,生成 COx 的主要途径包括通过表面和气相反应将 CH3 转化为 CH3O(s)/CH3O。气相反应还促进了 C2H4 的深度氧化。敏感性分析表明,甲烷的活化主要受表面反应的支配,而 CH3 与 C2H6 的耦合则主要由气相反应驱动。表面反应和气相反应对 C2H4 的形成具有同等作用。此外,压力依赖性分析表明,CH3 偶联反应的高压极限限制了 C2 产率随压力升高而增加。总之,本研究全面考察了 OCM 的规格池,建立了与实验结果相一致的微动力学模型,并阐明了由自由基驱动的反应网络。这些见解为优化反应条件和催化剂性能提供了宝贵的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


