DFT calculation-aided optimisation of a chiral phosphoric acid catalyst: case study of kinetic resolution of racemic secondary alcohols through lactonisation†
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chiral phosphoric acids (CPAs) with a pseudo C2 symmetric structure are privileged chiral Brønsted acid catalysts that have been used to accomplish challenging organic transformations in an enantioselective manner. However, it is sometimes difficult to improve enantioselectivity by exploring CPA catalysts experimentally. In order to demonstrate a case study to overcome this issue, we attempted to screen chiral backbones and substituents of CPAs by quantum chemical calculations for the kinetic resolution of racemic γ-hydroxy esters with a stereogenic centre at the γ-position through a lactonisation reaction. In constructing the theoretical prediction model, representative reaction pathways based on the two-step reaction mechanism of lactonisation were considered, and CPA candidates were screened rationally and efficiently without having to perform an exhaustive search for plausible reaction pathways by DFT (density functional theory) calculations. As a result, the selectivity factor (s-factor) was increased by using the predicted CPA with reduced computational load and experimental effort. This prediction model has a downside to be considered because some energetically unfavourable reaction pathways were ignored to simplify the model. However, the prediction model constructed on the basis of representative reaction pathways offers an efficient way to rapidly screen for catalysts and has been employed for rational catalyst optimisation while saving time and effort.
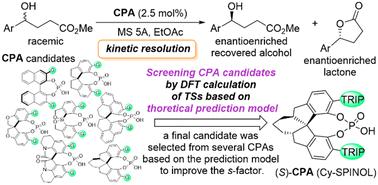
DFT 计算辅助优化手性磷酸催化剂:外消旋仲醇内酯化动力学解析案例研究†。
具有假 C2 对称结构的手性磷酸(CPA)是一种特殊的手性勃氏酸催化剂,已被用于以对映选择性的方式完成具有挑战性的有机转化。然而,有时很难通过实验探索 CPA 催化剂来提高对映选择性。为了展示克服这一问题的案例研究,我们尝试通过量子化学计算筛选 CPA 的手性骨架和取代基,用于通过内酯化反应动力学解析外消旋γ-羟基酯,该酯的立体中心位于γ 位。在构建理论预测模型时,考虑了基于内酯化两步反应机理的代表性反应途径,并合理有效地筛选出 CPA 候选化合物,而无需通过 DFT(密度泛函理论)计算对合理的反应途径进行详尽的搜索。结果,通过使用预测的 CPA,减少了计算负荷和实验工作量,提高了选择性因子(s-因子)。这种预测模型也有不足之处,因为为了简化模型,忽略了一些能量上不利的反应途径。不过,基于代表性反应途径构建的预测模型为快速筛选催化剂提供了一种有效方法,并已用于催化剂的合理优化,同时节省了时间和精力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


