Enantioselective Nickel-Electrocatalyzed Cross-Dehydrogenative α- and γ-Nitroalkylation
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Asymmetric catalytic versions of electricity-driven processes hold immense potential for the sustainable preparation of chiral compounds. However, the involvement of anodic oxidative cross-dehydrogenative coupling events between two distinct nucleophiles makes it challenging for a chiral catalyst to regulate the stereochemistry of the products. Our current electrocatalytic strategy for enantioconvergent cross-dehydrogenative α- and γ-nitroalkylation via radical-based pathways produces an array of enantioenriched nitroesters without supplementary stoichiometric oxidants. Mechanistic investigations reveal that the nickel catalyst plays a key role in both the electrochemical activation of the substrates and the stereoselectivity-defining events, affording the electrochemically generated Lewis acid-bound α-carbonyl radicals to interact with in situ-generated nitronate anions in a stereoselective manner. This electrocatalytic approach enables transformations that are highly challenging under thermal conditions, such as umpolung reactivity with readily available substrates, all-carbon quaternary stereocenter creation, and the control of remote stereochemistry.
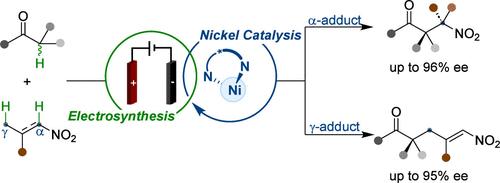
对映选择性镍电催化交叉脱氢α-和γ-硝基烷基化反应
电驱动过程的不对称催化版本在持续制备手性化合物方面具有巨大潜力。然而,由于两个不同的亲核物之间存在阳极氧化交叉脱氢偶联事件,因此手性催化剂调节产物的立体化学具有挑战性。我们目前的电催化策略是通过基于自由基的途径进行对映转化交叉脱氢α-和γ-硝基烷基化反应,在不需要补充化学计量氧化剂的情况下产生一系列对映富集的硝基酯。机理研究表明,镍催化剂在底物的电化学活化和立体选择性定义事件中都起着关键作用,使电化学生成的路易斯酸结合α-羰基自由基能以立体选择性的方式与原位生成的硝酸根阴离子相互作用。这种电催化方法实现了在热条件下极具挑战性的转化,例如与现成底物的umpolung 反应性、全碳四元立体中心的产生以及远程立体化学控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


