Methionine deprivation inhibits glioma proliferation and EMT via the TP53TG1/miR-96-5p/STK17B ceRNA pathway
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Recent research highlights the significant impact of methionine metabolism on glioma progression. An increasing amount of compelling evidence bridges long non-coding RNAs to abnormal metabolism in gliomas. However, the specific role of long non-coding RNAs in methionine metabolism regulating glioma progression remains unclear. This study reveals that methionine deprivation inhibits the proliferation, migration, and invasion capabilities of gliomas. Interestingly, the expression of TP53TG1, a long non-coding RNA, is also suppressed. TP53TG1 is highly expressed in gliomas and associated with poor patient outcomes. Subsequently, our data proves that inhibition of TP53TG1 suppresses glioma cell proliferation and the epithelial-mesenchymal transition process both in vitro and in vivo. Ultimately, we found that the underlying mechanism involves a competing endogenous RNA regulating network, in which TP53TG1 modulates the target protein STK17B by competitively binding to miR-96-5p, thus regulating glioma progression. These findings suggest that targeting methionine deprivation could be a promising approach for the clinical treatment of glioma.
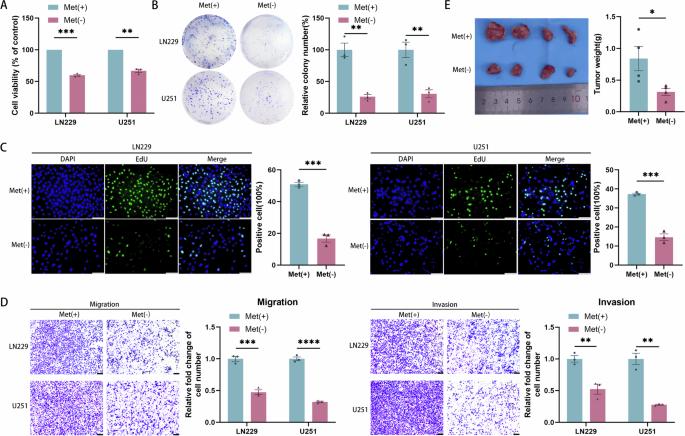
剥夺蛋氨酸可通过 TP53TG1/miR-96-5p/STK17B ceRNA 途径抑制胶质瘤的增殖和 EMT。
最近的研究突显了蛋氨酸代谢对胶质瘤进展的重大影响。越来越多令人信服的证据表明,长非编码 RNA 与胶质瘤代谢异常有关。然而,长非编码RNA在蛋氨酸代谢中调节胶质瘤进展的具体作用仍不清楚。本研究揭示了蛋氨酸的缺失会抑制胶质瘤的增殖、迁移和侵袭能力。有趣的是,长非编码 RNA TP53TG1 的表达也受到抑制。TP53TG1 在胶质瘤中高表达,与患者的不良预后有关。随后,我们的数据证明,抑制 TP53TG1 可以在体外和体内抑制胶质瘤细胞的增殖和上皮-间质转化过程。最终,我们发现其潜在机制涉及一个竞争性的内源性 RNA 调节网络,其中 TP53TG1 通过与 miR-96-5p 竞争性结合来调节靶蛋白 STK17B,从而调节胶质瘤的进展。这些研究结果表明,以蛋氨酸剥夺为靶点可能是一种很有前景的胶质瘤临床治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


