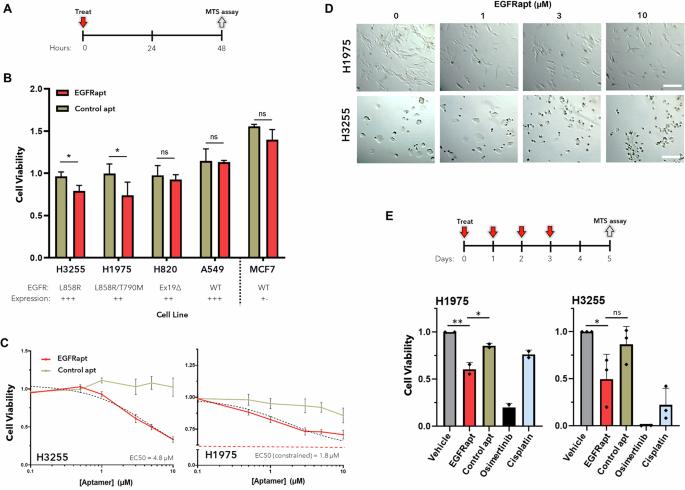
Anti-EGFR aptamer exhibits direct anti-cancer effects in NSCLC cells harboring EGFR L858R mutations
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Non-small cell lung cancer (NSCLC) adenocarcinoma (LUAD) is a leading cause of death worldwide. Activating mutations in the tyrosine kinase domain of the oncogene epidermal growth factor receptor (EGFR) are responsible for ~10–50% of all LUAD cases. Although tyrosine kinase inhibitors (TKIs) have been effective in prolonging patient survival and quality of life, acquired resistance and disease progression are inevitable, presenting a clear unmet need for alternative or adjuvant therapeutics. Here we show that an anti-EGFR aptamer (EGFRapt) decreases viability and tumor growth of LUAD cell lines harboring the L858R ± T790M mutation in EGFR. Additionally, we elucidate the mechanism by which EGFRapt exerts these effects by monitoring cellular processes associated with kinase-dependent and kinase-independent mechanisms. Overall, these data establish that EGFRapt has direct anti-cancer activity in mutant EGFR positive LUAD via targetable mechanisms that are independent of existing approaches, and they provide a foundation for further development of nucleic acid-based therapies that target EGFR.
抗表皮生长因子受体适配体在表皮生长因子受体 L858R 突变的 NSCLC 细胞中表现出直接的抗癌效果。
非小细胞肺癌(NSCLC)腺癌(LUAD)是导致全球死亡的主要原因。癌基因表皮生长因子受体(EGFR)酪氨酸激酶域的激活突变是导致约10%-50%的LUAD病例的原因。尽管酪氨酸激酶抑制剂(TKIs)能有效延长患者的生存期并提高生活质量,但获得性耐药和疾病进展不可避免,因此对替代或辅助疗法的需求明显未得到满足。在这里,我们展示了一种抗表皮生长因子受体适配体(EGFRapt)可降低携带表皮生长因子受体 L858R ± T790M 突变的 LUAD 细胞系的活力和肿瘤生长。此外,我们还通过监测与激酶依赖性和激酶非依赖性机制相关的细胞过程,阐明了 EGFRapt 发挥这些作用的机制。总之,这些数据证实了 EGFRapt 通过独立于现有方法的可靶向机制,对突变表皮生长因子受体阳性 LUAD 具有直接的抗癌活性,并为进一步开发靶向表皮生长因子受体的核酸疗法奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


