The immune checkpoint molecule B7-H4 regulates β-cell mass and insulin secretion by modulating cholesterol metabolism through Stat5 signalling
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
B7-H4 (B7S1, B7x, VTCN1) is an important immune checkpoint molecule that maintains immune homeostasis and is also expressed in pancreatic β cells. The polymorphism of B7-H4 influences the prevalence of Type 2 diabetes (T2D), suggesting a potential role of B7-H4 in the physiological function of pancreatic β cells and the pathogenesis of T2D.
Methods
β-cell-specific B7-H4 knockout mice (B7-H4 cKO mice) and their wild-type littermates were used to investigate the in vivo effects of B7-H4 on pancreatic β-cell morphology and function. AAV2/8-ins2-B7H4 and a control virus were infused via the pancreatic intraduct into high-fat diet (HFD)-treated mice to elucidate the therapeutic effect of B7-H4. RNA sequencing was conducted on primary islets. A Luminex assay was used to quantify cytokine changes in B7-H4 cKO mice. Electron microscopy imaging was used to observe insulin secretory vesicles in pancreatic β cells.
Results
Lesion of B7-H4 in β cells results in glucose intolerance due to reduced β-cell mass and deficient insulin secretion, whereas overexpression of B7-H4 in β cells ameliorates glucose intolerance in HFD-fed mice. Mechanistically, B7-H4 deficiency activates signal transducer and activator of transcription 5 (Stat5) signalling, which inhibits the expression of apolipoprotein F (Apof), leading to reduced cholesterol efflux and accumulated cholesterol in β cells, thereby impairing insulin processing and secretion. Overexpression of Apof in β cells or intraperitoneal injection of a Stat5 inhibitor reverses the metabolic phenotype and insulin secretion deficiency in B7-H4 cKO mice.
Conclusion
Our study demonstrated that B7-H4 plays an important role in regulating β-cell mass and insulin secretion, which may shed new light on the development of novel strategies for T2D treatment.
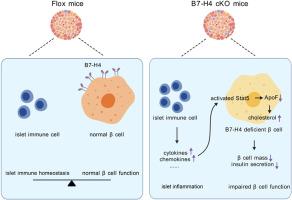
免疫检查点分子B7-H4通过Stat5信号调节胆固醇代谢,从而调节β细胞质量和胰岛素分泌。
背景:B7-H4(B7S1、B7x、VTCN1)是一种重要的免疫检查点分子,可维持免疫平衡,也可在胰腺β细胞中表达。方法:用β细胞特异性B7-H4基因敲除小鼠(B7-H4 cKO小鼠)及其野生型同窝小鼠研究B7-H4对胰腺β细胞形态和功能的体内影响。将AAV2/8-ins2-B7H4和对照病毒通过胰腺导管注入高脂饮食(HFD)小鼠体内,以阐明B7-H4的治疗效果。对原始胰岛进行了 RNA 测序。使用Luminex测定法量化B7-H4 cKO小鼠体内细胞因子的变化。电子显微镜成像用于观察胰腺β细胞中的胰岛素分泌泡:结果:β细胞中B7-H4的缺失会导致β细胞质量减少和胰岛素分泌不足,从而导致葡萄糖不耐受,而β细胞中B7-H4的过表达则会改善高密度脂蛋白喂养小鼠的葡萄糖不耐受。从机理上讲,B7-H4 缺乏会激活信号转导子和转录激活子 5(Stat5)信号,从而抑制载脂蛋白 F(Apof)的表达,导致胆固醇外流减少和胆固醇在 β 细胞中积累,从而影响胰岛素的加工和分泌。在β细胞中过表达Apof或腹腔注射Stat5抑制剂可逆转B7-H4 cKO小鼠的代谢表型和胰岛素分泌缺陷:我们的研究表明,B7-H4在调节β细胞质量和胰岛素分泌中发挥着重要作用,这可能为开发治疗T2D的新策略提供新的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


