AMPK regulates the maintenance and remodelling of the neuromuscular junction
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
The molecular mechanisms underlying the maintenance and adaptability of the neuromuscular junction (NMJ) remain poorly understood. This study aimed to investigate the role of AMP-activated protein kinase (AMPK) as a key regulator of NMJ stability and plasticity.
Method
A comprehensive, multifaceted approach was employed, integrating genetic, physiological, and pharmacological methodologies to elucidate the role of skeletal muscle AMPK in modulating the neuromuscular synapse.
Results
Our findings reveal an increased abundance of AMPK transcripts within the NMJ and an age-associated decline in AMPK activity and synapse-specific mitochondrial gene expression. Young mice null for skeletal muscle AMPK displayed a neuromuscular phenotype akin to aged animals. Pharmacological AMPK stimulation facilitated its localization in subsynaptic myonuclei, preceded the induction of several NMJ-related transcripts, and enhanced myotube acetylcholine receptor clustering. Exercise-induced AMPK activation in mouse muscle elicited a broad NMJ-related gene response, consistent with human exercise data.
Conclusions
These findings highlight a critical role for AMPK in the maintenance and remodeling of the NMJ, highlighting its potential as a therapeutic target for age-related and neuromuscular disorders.
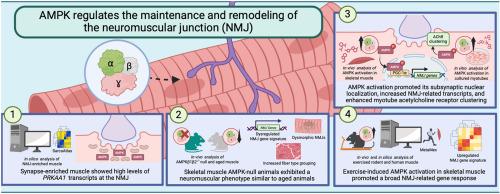
AMPK 调节神经肌肉接头的维持和重塑。
神经肌肉接头(NMJ)是一种电化学信号装置,对于促进肌肉收缩以及对抗与衰老和神经肌肉疾病相关的神经退行性过程至关重要。尽管我们对支配 NMJ 维护和可塑性的分子机制了解有限,但最近的证据表明,AMP 激活蛋白激酶(AMPK)是一个新兴的、有影响力的角色。我们的研究结果表明,AMPK 转录物在 NMJ 中的丰度增加,而 AMPK 活性和突触特异性线粒体基因表达的下降与年龄有关。骨骼肌AMPK无效的年轻小鼠表现出与老年动物相似的神经肌肉表型。药理 AMPK 刺激促进了其在突触下肌核中的定位,先于几种 NMJ 相关转录本的诱导,并增强了肌管乙酰胆碱受体的聚集。运动诱导的小鼠肌肉 AMPK 激活引起了广泛的 NMJ 相关基因反应,这与人类的运动数据一致。这些发现共同强调了 AMPK 在维持和重塑 NMJ 中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


