Itaconic acid ameliorates necrotizing enterocolitis through the TFEB-mediated autophagy-lysosomal pathway
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Excessive autophagy has been implicated in the pathogenesis of necrotizing enterocolitis (NEC), yet the molecular underpinnings of the autophagy-lysosomal pathway (ALP) in NEC are not well characterized. This study aimed to elucidate alterations within the ALP in NEC by employing RNA sequencing on intestinal tissues obtained from affected infants. Concurrently, we established animal and cellular models of NEC to assess the therapeutic efficacy of itaconic acid (ITA). Our results indicate that the ALP is significantly disrupted in NEC. Notably, ITA was found to modulate the ALP, enhancing autophagic flux and lysosomal function, which consequently alleviated NEC symptoms. Further analysis revealed that ITA's beneficial effects are mediated through the promotion of TFEB nuclear translocation, thereby augmenting the ALP. These findings suggest that targeting the ALP with ITA to modulate TFEB activity may represent a viable therapeutic approach for NEC.
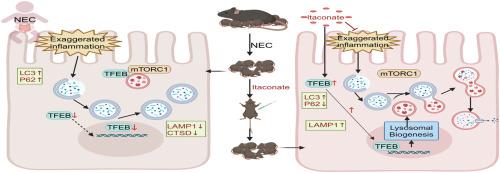
衣康酸通过 TFEB 介导的自噬-溶酶体途径改善坏死性小肠结肠炎。
自噬过多被认为与坏死性小肠结肠炎(NEC)的发病机制有关,但NEC中自噬-溶酶体通路(ALP)的分子基础尚不清楚。本研究旨在通过对受影响婴儿的肠道组织进行 RNA 测序,阐明 NEC 中 ALP 的变化。同时,我们还建立了 NEC 的动物和细胞模型,以评估衣康酸 (ITA) 的疗效。我们的研究结果表明,ALP 在 NEC 中受到严重破坏。值得注意的是,ITA能调节ALP,增强自噬通量和溶酶体功能,从而缓解NEC症状。进一步的分析表明,ITA 的有益作用是通过促进 TFEB 核转位来实现的,从而增强了 ALP。这些研究结果表明,用ITA靶向ALP来调节TFEB的活性可能是治疗NEC的一种可行方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
文献相关原料
公司名称
产品信息
索莱宝
DAPI
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


