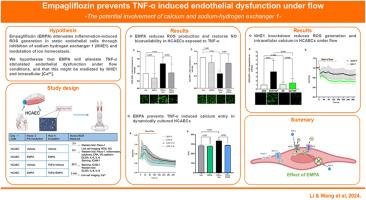
Empagliflozin prevents TNF-α induced endothelial dysfunction under flow -the potential involvement of calcium and sodium-hydrogen exchanger
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background
Empagliflozin (EMPA) attenuates inflammation-induced ROS generation in static endothelial cells through inhibition of sodium hydrogen exchanger 1 (NHE1) and modulation of ion homeostasis. We hypothesize that EMPA will alleviate TNF-α stimulated endothelial dysfunction under flow conditions, and that this might be mediated by NHE1 and intracellular Ca2+.
Methods
Human coronary artery endothelial cells were pre-treated with EMPA or vehicle before starting flow with or without TNF-α. Intracellular Ca2+ was recorded for 5 min at the start of flow. ROS generation and NO bioavailability, Piezo-1, cytokines, adhesion molecules, VE-cadherin and eNOS were detected after 6 h. BAPTA-AM was applied to chelate intracellular Ca2+ and NHE1 was knocked down with specific siRNA.
Results
Under flow conditions, EMPA inhibited ROS production and [Ca2+] increase in cells exposed to TNF-α (P < 0.05). BAPTA-AM and NHE1 knockdown both reduced ROS generation (P < 0.05), and genetical inhibition of NHE1 led to reduction of intracellular [Ca2+] in HCAECs receiving TNF-α (P < 0.05). Yet, EMPA showed no effect on the increased cytokine production, adhesion molecule expression and phosphorylation of eNOS in endothelial cells exposed to TNF-α.
Conclusion
EMPA mitigates increased ROS production and impaired NO bioavailability in TNF-α stimulated cells under flow. The anti-oxidative effect of EMPA is mediated by the decreased intracellular [Ca2+] following NHE1 inhibition.
恩格列净可预防TNF-α诱导的流动性内皮功能障碍--钙和钠-氢交换器的潜在参与。
背景:恩格列净(EMPA)通过抑制钠氢交换子1(NHE1)和调节离子平衡来减轻炎症诱导的静态内皮细胞ROS生成。我们假设,在流动条件下,EMPA 将缓解 TNF-α 刺激的内皮功能障碍,而这可能是由 NHE1 和细胞内 Ca2+ 介导的。在开始流动的 5 分钟内记录细胞内 Ca2+。6 小时后检测 ROS 生成和 NO 生物利用率、Piezo-1、细胞因子、粘附分子、VE-cadherin 和 eNOS:结果:在流动条件下,EMPA抑制了暴露于TNF-α的细胞中ROS的产生和[Ca2+]的增加:EMPA可缓解血流中TNF-α刺激细胞产生的ROS增加和NO生物利用率的降低。EMPA的抗氧化作用是由NHE1抑制后细胞内[Ca2+]的减少所介导的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


