Exploring bile acid transporters as key players in cancer development and treatment: Evidence from preclinical and clinical studies
IF 9.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Bile acid transporters (BATs) are integral membrane proteins belonging to various families, such as solute carriers, organic anion transporters, and ATP-binding cassette families. These transporters play a crucial role in bile acid transportation within the portal and systemic circulations, with expression observed in tissues, including the liver, kidney, and small intestine. Bile acids serve as signaling molecules facilitating the absorption and reabsorption of fats and lipids. Dysregulation of bile acid concentration has been implicated in tumorigenesis, yet the role of BATs in this process remains underexplored. Emerging evidence suggests that BATs may modulate various stages of cancer progression, including initiation, development, proliferation, metastasis, and tumor microenvironment regulation. Targeting BATs using siRNAs, miRNAs, and small compound inhibitors in preclinical models and their polymorphisms are well-studied for transporters like BSEP, MDR1, MRP2, OATP1A2, etc., and have shed light on their involvement in tumorigenesis, particularly in cancers such as those affecting the liver and gastrointestinal tract. While BATs' role in diseases like Alagille syndrome, biliary atresia, and cirrhosis have been extensively studied, their implications in cancer warrant further investigation. This review highlights the expression and function of BATs in cancer development and emphasizes the potential of targeting these transporters as a novel therapeutic strategy for various malignancies.
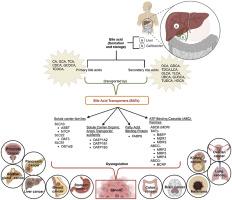
探索胆汁酸转运体在癌症发展和治疗中的关键作用:来自临床前和临床研究的证据
胆汁酸转运体(BATs)是一种整体膜蛋白,属于不同的家族,如溶质载体、有机阴离子转运体和 ATP 结合盒家族。这些转运体在门静脉和全身循环中的胆汁酸运输中起着至关重要的作用,在肝脏、肾脏和小肠等组织中均有表达。胆汁酸是促进脂肪和脂质吸收和再吸收的信号分子。胆汁酸浓度失调与肿瘤发生有关,但胆汁酸在这一过程中的作用仍未得到充分探索。新的证据表明,胆汁酸可调节癌症进展的各个阶段,包括起始、发展、增殖、转移和肿瘤微环境调控。在临床前模型中使用 siRNAs、miRNAs 和小化合物抑制剂靶向 BATs,并对 BSEP、MDR1、MRP2、OATP1A2 等转运体的多态性进行了深入研究,揭示了它们参与肿瘤发生的情况,尤其是在影响肝脏和胃肠道的癌症中。虽然 BATs 在阿拉吉尔综合征、胆道闭锁和肝硬化等疾病中的作用已被广泛研究,但它们在癌症中的影响还需要进一步研究。本综述重点介绍了 BATs 在癌症发展过程中的表达和功能,并强调了以这些转运体为靶点作为各种恶性肿瘤的新型治疗策略的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


