Functional characterization of Staphylococcus aureus lipase 2 (SAL2) as a collagen adhesin
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Extracellular lipases of many pathogens have been characterized as human virulence factors. Staphylococcus aureus produces a variety of enzymes that aid in the pathogenesis of the bacterium to invade and destroy host tissues, resulting in a wide range of clinical illnesses. The lipase is one such enzyme, and the lipases produced by S. aureus (SAL1, SAL2 and SAL3) have been associated with the virulence of the bacterium. In the present study, we cloned, expressed and purified the mature lipase domain of SAL2 (rSAL2296–690) and characterized its interaction with human collagen type IV using biolayer interferometry (BLI), molecular docking and simulation studies. Collagen binds to rSAL2296–690 with an affinity of 3.261 μM. The enzymatic activity of rSAL2296–690 was analyzed in the presence of collagen and orlistat, a potent lipase inhibitor. The activity of rSAL2296–690 in the presence of collagen or orlistat was nearly 90 fold lower than that of the native rSAL2296–690. The optimal pH and temperature for rSAL2296–690 activity were found at 7 and 25 °C respectively. rSAL2296–690 found to be stable at pH 7 and exhibits thermostability in the temperature range 15–25 °C. With CaCl2 and ZnCl2, an increase in activity of rSAL2296–690 was observed whereas NiSO4, CuSO4, MnCl2, CoCl2 and MgCl2 reduced the activity. No substrate specificity was found with rSAL2296–690, as it cleaves different substrate lengths (C2, C6, C12 and C16) and triglyceride triolein. Interaction of rSAL2296–690 with metal-incubated collagen revealed that the binding affinity of collagen in the presence of NiSO4, CuSO4 and CoCl2 significantly reduced. Enzymatic and collagen binding studies provided insights into the putative collagen binding site on SAL2296–690, which is near the active site region of the molecule. This study thus revealed that rSAL2296–690 as a bi-functional molecule, acts not only as a lipase but also as a collagen adhesin.
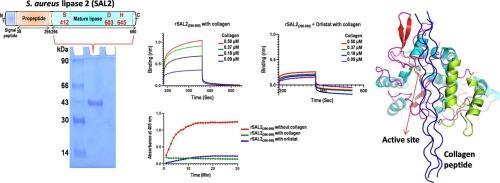
金黄色葡萄球菌脂肪酶 2(SAL2)作为胶原粘附蛋白的功能特征。
许多病原体的胞外脂肪酶已被定性为人类毒力因子。金黄色葡萄球菌会产生多种酶,这些酶有助于细菌入侵和破坏宿主组织的致病机理,从而导致多种临床疾病。脂肪酶就是这样一种酶,金黄色葡萄球菌产生的脂肪酶(SAL1、SAL2 和 SAL3)与该细菌的毒力有关。在本研究中,我们克隆、表达和纯化了 SAL2 的成熟脂肪酶结构域(rSAL2296-690),并利用生物层干涉测量法(BLI)、分子对接和模拟研究鉴定了其与人类 IV 型胶原蛋白的相互作用。胶原与 rSAL2296-690 的亲和力为 3.261 μM。在胶原蛋白和强效脂肪酶抑制剂奥利司他的存在下,对 rSAL2296-690 的酶活性进行了分析。在胶原蛋白或奥利司他存在下,rSAL2296-690 的活性比原生 rSAL2296-690 低近 90 倍。rSAL2296-690 在 pH 值为 7 时稳定,在 15-25 °C 的温度范围内具有热稳定性。在使用 CaCl2 和 ZnCl2 时,观察到 rSAL2296-690 的活性增加,而使用 NiSO4、CuSO4、MnCl2、CoCl2 和 MgCl2 时活性降低。由于 rSAL2296-690 能裂解不同长度的底物(C2、C6、C12 和 C16)和甘油三酯三油脂,因此它没有底物特异性。rSAL2296-690 与金属培养的胶原蛋白的相互作用表明,在 NiSO4、CuSO4 和 CoCl2 存在下,胶原蛋白的结合亲和力显著降低。酶切和胶原蛋白结合研究揭示了 SAL2296-690 上的胶原蛋白结合位点,该位点靠近分子的活性位点区域。因此,这项研究揭示了 rSAL2296-690 作为一种双功能分子,不仅可以作为脂肪酶,还可以作为胶原蛋白粘合剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


