The RNA Silencing Suppressor P8 From High Plains Wheat Mosaic Virus is a Functional Tetramer
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
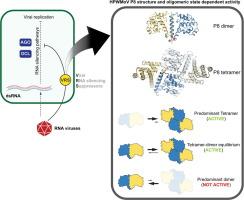
In plants, RNA interference (RNAi) serves as a critical defense mechanism against viral infections by regulating gene expression. However, viruses have developed RNA silencing suppressor (RSS) proteins to evade this defense mechanism. The High Plains wheat mosaic virus (HPWMoV) is responsible for the High Plains disease in wheat and produces P7 and P8 proteins, which act as RNA silencing suppressors. P8, in particular, lacks sequence similarity to known suppressors, prompting inquiries into its structure and function.
Here, we present a comprehensive analysis of P8, elucidating its structure and function. Using X-ray crystallography, we resolved the full-length P8 structure at 1.9 Å resolution, revealing a tetrameric arrangement formed by two identical dimers. Through structure-based mutagenesis, biochemical assays, and functional studies in plants, we demonstrate that HPWMoV P8’s RNA silencing suppression activity relies on its oligomeric state.
Contrary to previous report, our findings indicate that while a P8 fused to maltose-binding protein (MBP-P8) was hypothesized to bind short double-stranded RNA, the native P8 tetramer does not interact with small interfering RNA (siRNA). This suggests an alternative mechanism for its function, yet to be determined.
Our study sheds light on the structural and functional characteristics of HPWMoV P8, providing valuable insights into the complex interplay between viral suppressors and host defense mechanisms.
Significance statement
Effective action to address malnutrition in all its forms requires an understanding of the mechanisms affecting it. Wheat, crucial for human and animal consumption, faces threats from biotic and abiotic stresses. RNA silencing is a key defense against viral infections in plants. Plant viruses employ various mechanisms, including encoding viral RNA silencing suppression (VRS) proteins, to evade host immune responses. Despite the conservation of RNA-silencing pathways, viral RSS proteins exhibit diverse sequences, structures, and mechanisms. Our study focuses on P8, an RSS protein from HPWMoV. Understanding its structure and assembly is a crucial step toward comprehending how these viruses counteract host defenses, aiding in combatting malnutrition.
高原小麦花叶病毒的 RNA 沉默抑制因子 P8 是一种功能性四聚体。
在植物中,RNA 干扰(RNAi)是通过调节基因表达来抵御病毒感染的重要防御机制。然而,病毒已经开发出 RNA 沉默抑制蛋白(RSS)来逃避这种防御机制。高原小麦花叶病毒(HPWMoV)是小麦高原病的罪魁祸首,它产生的 P7 和 P8 蛋白是 RNA 沉默抑制因子。尤其是 P8,它与已知的抑制因子缺乏序列相似性,这促使人们对其结构和功能进行研究。在这里,我们对 P8 进行了全面分析,阐明了它的结构和功能。通过 X 射线晶体学,我们以 1.9 Å 的分辨率解析了 P8 的全长结构,揭示了由两个相同的二聚体形成的四聚体排列。通过基于结构的诱变、生化实验和植物功能研究,我们证明了 HPWMoV P8 的 RNA 沉默抑制活性依赖于其低聚物状态。与之前的报告相反,我们的研究结果表明,虽然假定与麦芽糖结合蛋白(MBP-P8)融合的 P8 能结合短双链 RNA,但原生 P8 四聚体并不能与小干扰 RNA(siRNA)相互作用。这表明其功能的另一种机制尚待确定。我们的研究揭示了 HPWMoV P8 的结构和功能特征,为了解病毒抑制剂与宿主防御机制之间复杂的相互作用提供了宝贵的见解。意义声明 要有效解决各种形式的营养不良问题,就必须了解影响营养不良的机制。小麦对人类和动物的食用至关重要,它面临着来自生物和非生物胁迫的威胁。RNA 沉默是植物抵御病毒感染的关键防御手段。植物病毒利用各种机制,包括编码病毒 RNA 沉默抑制(VRS)蛋白,来逃避宿主的免疫反应。尽管 RNA 沉默途径保持不变,但病毒 RSS 蛋白的序列、结构和机制却各不相同。我们的研究重点是 P8,它是 HPWMoV 的一种 RSS 蛋白。了解它的结构和组装是理解这些病毒如何对抗宿主防御、帮助对抗营养不良的关键一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


